Fig. 5.
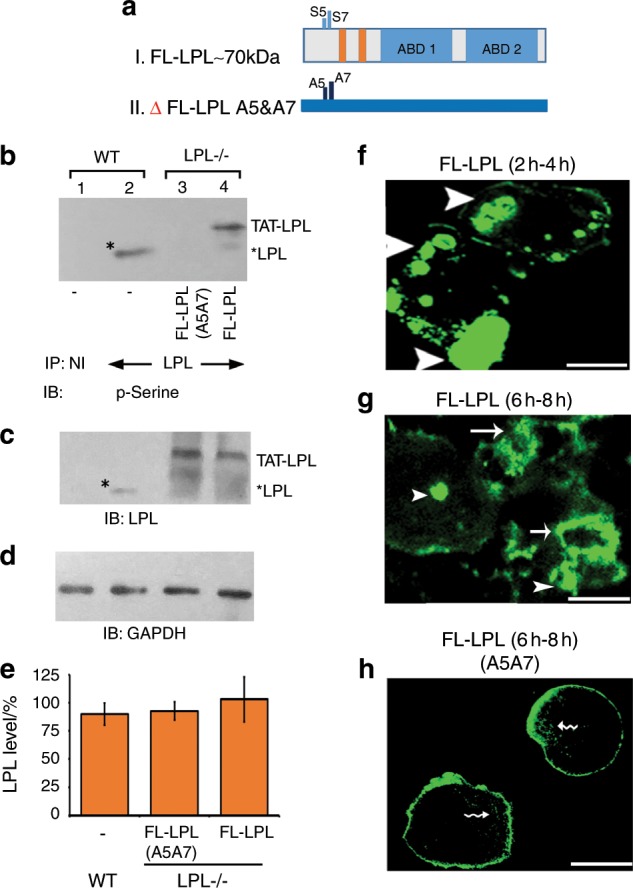
Intermittent time-lapse video microscopy analyses in LPL−/− OCs transduced with TAT-fused FL-LPL peptides. a Schematic diagram demonstrating the structure of FL-LPL (I) and mutated (Δ) FL-LPL (A5A7) (II) peptides. b–e Immunoblotting (IB) analysis: immunoprecipitates made with an antibody to LPL (lanes 2–3) or nonimmune serum (NI; lane 1) were immunoblotted with an antibody to phosphoserine (p-Serine; b). Stripping and reprobing with an LPL antibody is shown in c. Asterisks indicate endogenous LPL in WT osteoclasts (b, c). Equal amount of lysate protein (Input) used for the immunoprecipitation was assessed by direct IB of the lysates with a GAPDH antibody (d). Transduced protein levels from three different experiments are shown as a graph in e (error bar represents SD). Data were assessed by standard Student’s t test. There is no statistically significant difference in the levels of LPL protein between groups. f–h Osteoclasts expressing GFP-actin were transduced with TAT-fused FL-LPL (f, g) or FL-LPL (A5A7; h) for 15 min; then plated on dentine slices and supplemented with TNF-α. Analyses were done at 2 h–4 h (f) and 6 h–8 h (g, h). NSZs are indicated by arrowheads in f and g. Single and multiple sealing rings (indicated by arrows) are observed at 6 h–8 h in LPL−/− osteoclasts transduced with FL-LPL (g). Actin distribution was observed in podosomes (indicated by wavy arrows) and the plasma membrane in LPL−/− osteoclasts transduced with ΔFL-LPL (A5A7). The results shown are representative of three different experiments from three different osteoclasts preparations. Scale bar—25 μm.
