Figure 4.
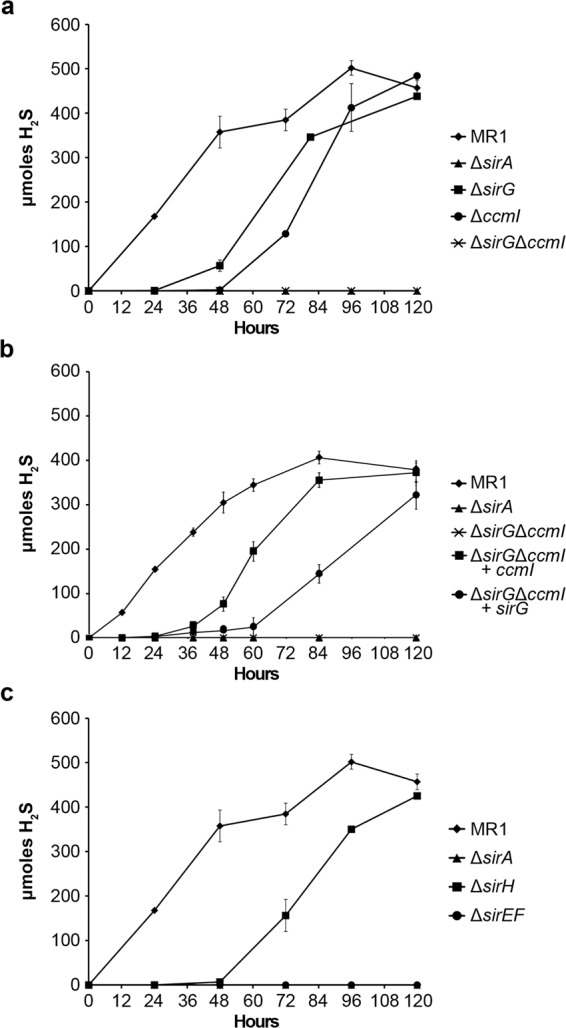
Cytochrome maturation and heme lyase components are required for optimal sulfite reduction. Wildtype and mutant S. oneidensis were grown anaerobically with sulfite as the sole electron acceptor. Sulfite reduction was indicated by the production of hydrogen sulfide (H2S). (a) Loss of either heme chaperone, SirG or CcmI, resulted in a lag in sulfite reduction, likely due to a reduction in functionally active reductase. Sulfite activity was abolished when both sirG and ccmI were deleted. (b) Sulfite reductase activity of the ΔsirGΔccmI double mutant was partially restored, similar to that of the respected single mutant, when complemented with either sirG or ccmI. (c) Loss of the heme lyase components SirH or SirEF resulted in decreased sulfite reduction. The ΔsirH mutant exhibited a 48-hour lag in sulfite reduction compared to wildtype, whereas the ΔsirEF mutant lacked any sulfite reduction. The ΔsirA mutant served as a negative control for sulfite reduction in all assays.
