Figure 2.
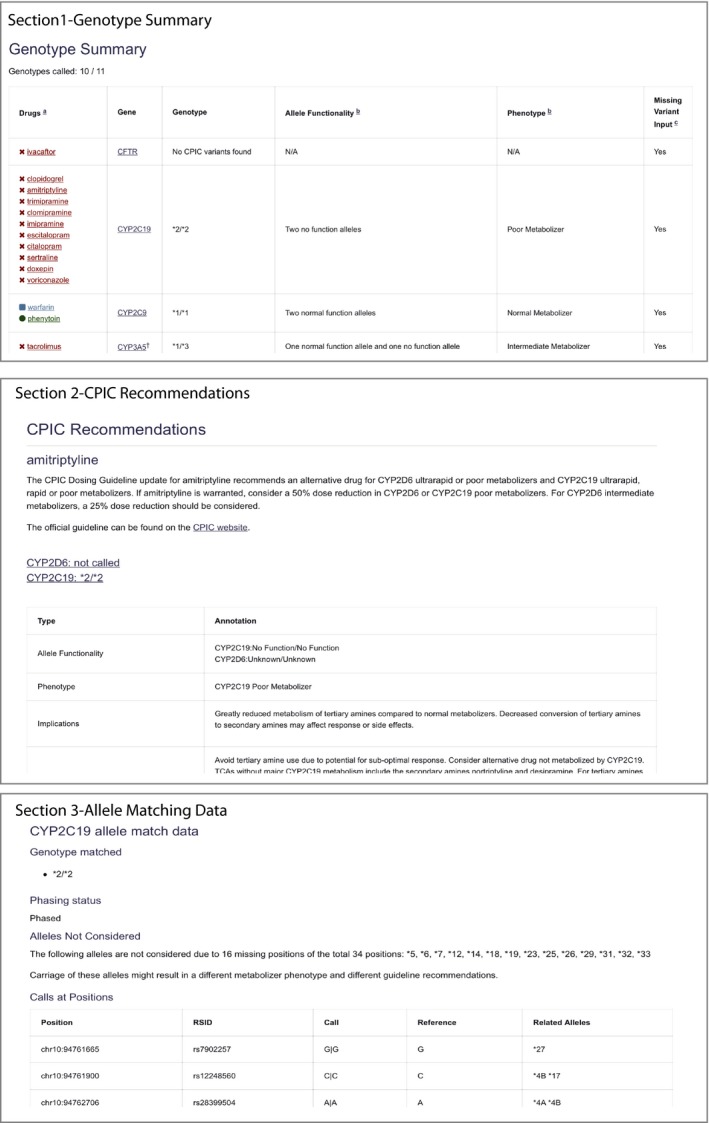
PharmCAT report sections for the 1000 Genomes VCF file for Coriell sample NA12717. The PharmCAT report consists of four parts: (i) genotype summary table, (ii) Clinical Pharmacogenetics Implementation Consortium (CPIC) recommendations section by drug in alphabetical order, (iii) gene information about the interrogated variants, and (iv) disclaimer. In the summary table, the drugs are colored to indicate whether CPIC recommends a prescribing change based on the given genotype. The last column in the table indicates star alleles that could not be considered for the genotype assignment due to missing variant information in the VCF file. It is important to note missing information because it could result in changes to the phenotype and/or CPIC recommendation. PharmCAT, pharmacogenomics clinical annotation tool. [Colour figure can be viewed at https://www.wileyonlinelibrary.com]
