Abstract
Background
Compound Kushen injection (CKI) is a traditional Chinese medicine preparation for clinical treatment of cancer pain or treatment of various types of solid tumors. The purpose of this study was to identify the main active compounds from CKI and to investigate its anti-cancer mechanisms via drug target biological network pharmacology construction and prediction.
Material/Methods
Constituents of CKI were retrieved from Traditional Chinese Medicine Systems Pharmacology (TCMSP) database. Disease targets were collected in the Human Gene (Gene Cards) and Human Mendelian Inheritance (OMIM) databases. “Ingredients-protein targets-pathway” networks were constructed using Cytoscape. STRING database platform to construct enrichment of protein-protein interactions (PPI), related diseases and pathways network. Gene Ontology (GO) biological functions and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway of were performed to investigate by using Bioconductor tool for analysis.
Results
The results indicated that 60 constituents of absorption, distribution, metabolism, and excretion (ADME) filtration resulted in 33 constituents exhibiting significant correlations with anti-cancer and CKI may target 113 proteins, including IL6, EGFR, CASP3, VEGFA, MYC, and ESR1. GO and KEGG enrichment analysis results show that 129 biological processes and 93 signal pathways associated with cancer. It mainly involves cancers such as prostate cancer, bladder cancer, hepatocellular carcinoma, colorectal cancer, breast cancer, etc. Active ingredients might also induce apoptosis in cancer cells via the p53 and PI3K-Akt signaling pathway mechanism.
Conclusions
This study was based on pharmacological networks results for the prediction of the multi-constituent, multi-target, and multi-pathway mechanisms of CKI, which might be a promising potential therapeutic and prevention candidate for anti-cancer. However, based on computer data mining and analysis, this study still needs to be further verified by in vivo/in vitro experiments, and the safety of CKI needs to be evaluated.
MeSH Keywords: Antineoplastic Agents; Ethnopharmacology; Medicine, Chinese Traditional; Protein Interaction Maps; Protein Transport
Background
Nowadays, cancer is considered among one of the leading causes of human death in the world, which creates a severe economic burden on society and the family. According to the latest statistics from the International Agency for Research on Cancer (IARC), the incidence and mortality of cancer are increasing rapidly around the world. Although the huge advancement has evolved in the treatment of cancer by surgery, radiotherapy, chemotherapy, and immunotherapy in the past decades, the discovery of more effective and less toxic cancer treatments is a highly active area of research [1].
Compound Kushen injection (CKI, commercial name Yan Shu) is mainly used to treat cancer-related pain. CKI consists of 2 main herbs, Kushen (Sophorae Flavescentis Radix) and Baituling (Smilacis Glabrae Rhixoma). There are many ingredients in the Chinese herbal medicine Sophorae Flavescentis Radix, with matrine and oxymatrine the 2 main components, which can exert anti-inflammatory, anti-tumor, anti-viral, and cardiovascular protection effects [2,3]. Recent evidence shows that Smilacis Glabrae Rhixoma also produces anti-cancer actions, such as it beng reported to effectively suppress the phosphorylation of Akt (Thr308), thereby inhibiting gastric cancer cell proliferation and metastasis and accelerating its apoptosis through Akt-mediated signaling pathways [4]. This indicates that the components of both major drugs in CKI can exert anti-cancer effects through multiple biological processes or multi-target related pathways.
In the study of traditional Chinese medicine compound preparations and monomers, network pharmacology has been widely used in China, including data collection, target prediction, network analysis of multi-component interactions and signal path prediction. The screening of multi-target of traditional Chinese medicines, the synergy between each active ingredient and the joint application of traditional Chinese medicines and marketed drugs are the focus of research [5]. Traditional Chinese medicine preparations contain many active ingredients, the mechanism of action is complicated. It is necessary to establish a network pharmacology method for traditional Chinese medicines, mainly based on systemic and molecular levels, to discover the active ingredients present in traditional Chinese medicines, to elucidate the mechanism of action of each active ingredient and to predict the target genes and pharmacological effects of these ingredients [6]. Network pharmacology emphasizes the integration of biological networks with drug action networks, to analyzes the relationship of the active ingredients of the drug and disease-associated genes by nodes or network modules in the network, from finding a single ingredients to a comprehensive network analysis to further clarify the anti-cancer mechanism of each active ingredient of the drug [7]. Conversely, researchers can predict whether a pharmaceutical preparation containing such a monomer has anti-cancer properties by discovering the anti-cancer effect of the monomer.
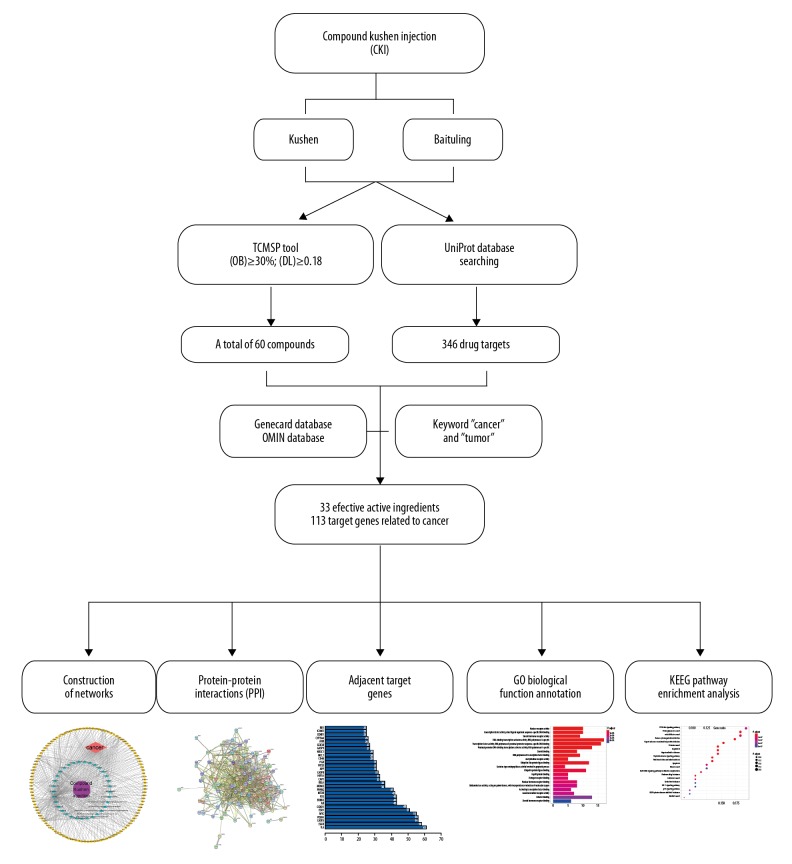
Although there have been many reports on anti-cancer studies of CKI, its interactive “Active ingredients-protein targets-signaling pathway” and the related anti-cancer molecular mechanisms remain unclear. Therefore, in this study, the active ingredients of CKI were screened by network pharmacology and the network of protein interactions was constructed to analyze its biological processes and mediated signaling pathways, which provided a reference for further anti-cancer research of CKI. Figure 1 illustrated network pharmacology analysis workflow.
Figure 1.
Workflow of the network pharmacology analysis of compound Kushen injection (CKI) constituents against cancer targets.
Material and Methods
Chemical ingredients database and active compounds screening
According to the keywords “Sophorae Flavescentis Radix” and “Smilacis Glabrae Rhixoma”, all chemical constituents of 2 individual herbs in CKI were retrieved from the Traditional Chinese Medicine Systems Pharmacology Database and Analysis Platform (TCMSP, http://lsp.nwu.edu.cn/) and related literature. The active constituents of CKI were screened by applying the absorption, distribution, metabolism, and excretion (ADME) criteria in pharmacokinetics, including oral bioavailability (OB) ≥30% and drug-likeness (DL) ≥0.18 [8,9]. The OB value mainly reflects the proportion of the drug absorbed into the body circulation by the human body, and the DL value mainly indicates the similarity between the compound and the known drug in the component, and both provide an important reference for the collection of active ingredients [10,11].
Disease-associated targets
We collected different gene targets associated with a keyword “cancer” and “tumor” from the Online Mendelian Inheritance in Man (OMIM, http://www.ncbi.nlm.nih.gov/omim) database and the Human Gene Database (GeneCards, http://www.genecards.org). Obtained targets were imported into UniProt (http://www.uniprot.org/) to search their information, including name, functions, symbol, and gene ID [16]. All known gene targets are cataloged, providing a basis for further research and analysis of the common target of “drug-disease”.
Construction of networks
The screened pharmaceutically active ingredients were introduced into the “drug-disease” common target gene, and the visual composition network of the active ingredient-cancer targets was constructed by Cytoscape 3.7.1 (http://www.cytoscape.org/) software. In the network diagram, the node represents the active ingredients of CKI and gene protein, the relationship between the active ingredient and the target of action was encoded by edges. In addition, the importance of the interaction between the nodes is determined by the number of lines connecting the target genes in the network.
Construction of target proteins interaction
The “drug-disease” target was imported into the STRING biological database (https://string-db.org/) for studying target protein interactions, also known as protein-protein interactions (PPI), and all the targets were only limited the species as “Homo sapiens”. In the PPI diagram, each solid circle represents a gene, and the middle of the circle shows the structure of the protein, while the circles are connected by lines of different colors. Each line represents the biological process between protein and protein, including regulation of gene expression, signal transduction, cell migration and so on.
Enrichment analysis for target proteins
Bioconductor 3.8 (http://www.bioconductor.org/) provides tools for the analysis and comprehension of high-throughput genomic data, which mainly using the R statistical programming language to development and process bioinformatics data. Target genes were utilized for the Gene Ontology (GO) enrichment analysis and the Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways using Bioconductor, thereby extracting the biological function and cancer-related pathways of the target proteins. CKI was predicted to exert anti-cancer effects by regulating the biological function of the target protein or related signaling pathways. Among them, the lower the P value, the more prominent the correlation between the target protein and cancer, and the threshold of P<0.05 was regarded as significant difference.
Results
Screening of active ingredients and related targets
In this study, TCMSP compound data were obtained from databases such as DrugBank, HIT, TTD, PharmGKB, etc. It is known that CKI is mainly composed of 2 herbs, Kushen and Baituling. Through the TCMSP database search, 113 ingredients and 963 related targets of Kushen, 74 ingredients of Baituling and 904 related targets were obtained. A total of 60 compounds were obtained from the TCMSP database using OB ≥30% and DL ≥0.18 as screening conditions. Convert the corresponding gene name into Gene Symbol through the UniProt database, delete duplicate and non-human-derived targets, and finally get 346 drug targets.
Prediction of anti-cancer targets of active ingredients
A total of 26 363 target genes related to cancer were collected from the Genecards database and the OMIM database, of which 26 087 targets were obtained from the Genecards database and 276 targets were mined in the OMIM database. After integrating and deleting the duplicated genes, 33 effective active ingredients were obtained (Table 1). Most of the active ingredients isolated from CKI include quercetin, luteolin, beta-sitosterol, naringenin, phaseolin, stigmasterol, formononetin, diosgenin, glyceollin and wighteone. These ingredients possess potent pharmacological activity. For example, quercetin, luteolin, beta-sitosterol, and naringenin are all-natural flavonoids with good pharmacological activity in terms of antioxidant, anti-inflammatory and anti-cancer activities [13–16]. A large number of in vivo or in vitro studies have demonstrated that the major active ingredients in CKI have anti-cancer properties. For example, phaseolin has an inhibitory effect on hepatoma cell line H4IIE [17]. Stigmasterol has an apoptosis-inducing effect on liver cancer (HepG2) cells [18]. Formononetin targets the FGFR2-mediated Akt signaling pathway, thereby inhibiting tumor growth and angiogenesis [19].
Table 1.
Active ingredients and ADME (absorption, distribution, metabolism, and excretion) parameters of Kushen and Baituling.
| Mol ID | Molecule name | Structure | BO(%) | DL | Herb |
|---|---|---|---|---|---|
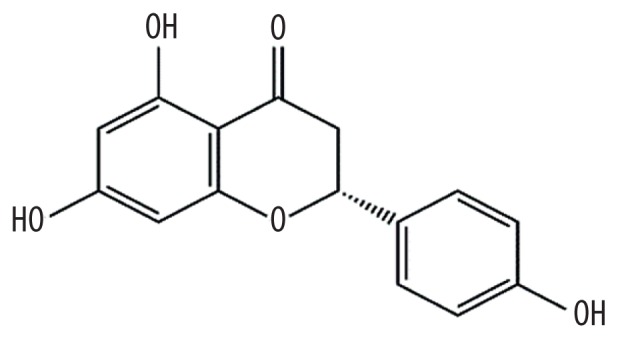
| MOL001040 | (2R)-5,7-dihydroxy-2-(4-hydroxyphenyl)chroman-4-one |
|
42.36 | 0.21 | Kushen |
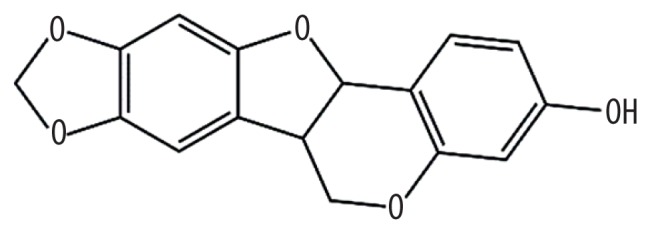
| MOL001484 | Inermine |
|
75.18 | 0.54 | Kushen |
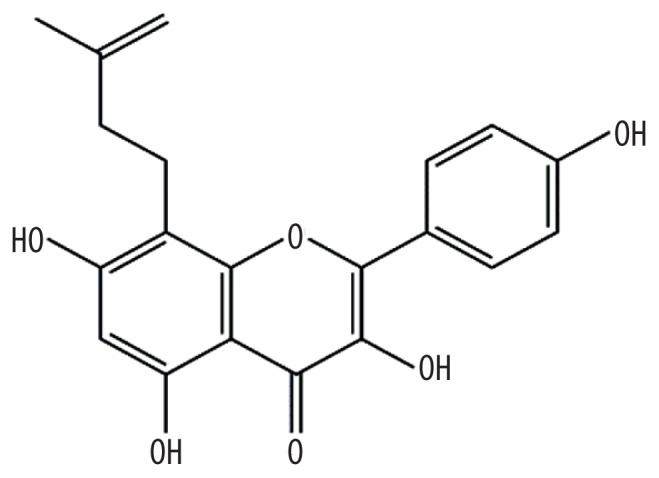
| MOL003542 | 8-isopentenyl-kaempferol |
|
38.04 | 0.39 | Kushen |
| MOL003627 | Sophocarpine |
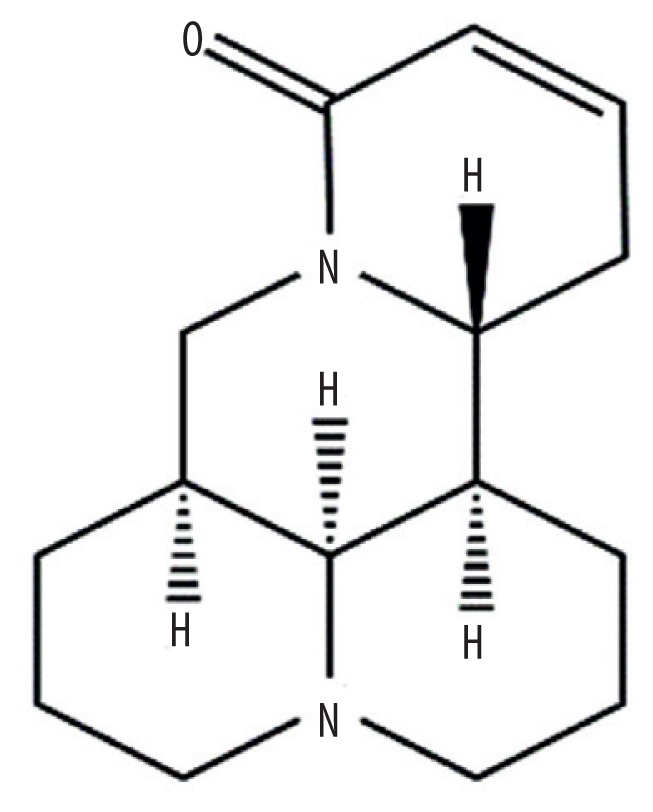
|
64.26 | 0.25 | Kushen |
| MOL003648 | Inermin |
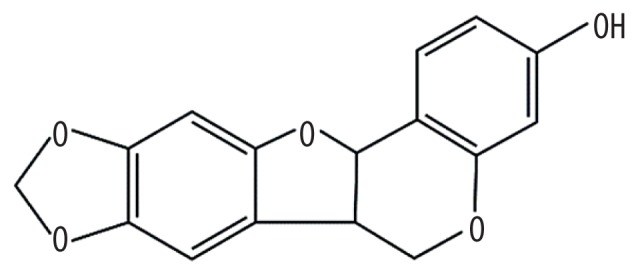
|
65.83 | 0.54 | Kushen |
| MOL003673 | Wighteone |
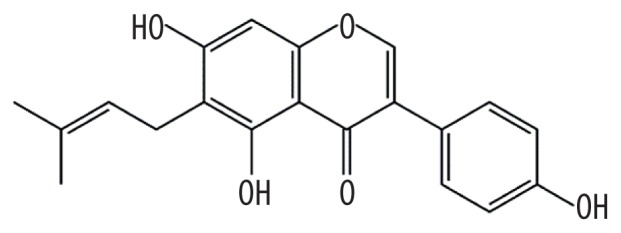
|
42.8 | 0.36 | Kushen |
| MOL003680 | Sophoridine |
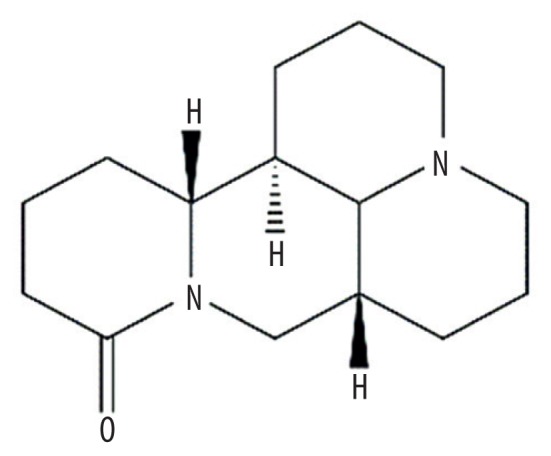
|
60.07 | 0.25 | Kushen |
| MOL000392 | Formononetin |
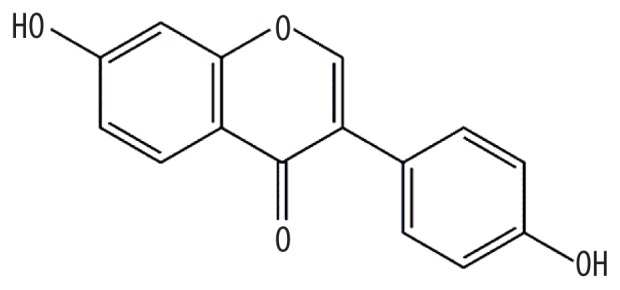
|
69.67 | 0.21 | Kushen |
| MOL004580 | cis-Dihydroquercetin |
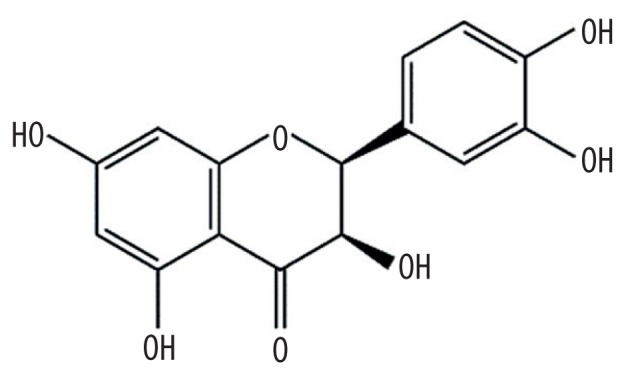
|
66.44 | 0.27 | Kushen or Baituling |
| MOL004941 | (2R)-7-hydroxy-2-(4-hydroxyphenyl)chroman-4-one |
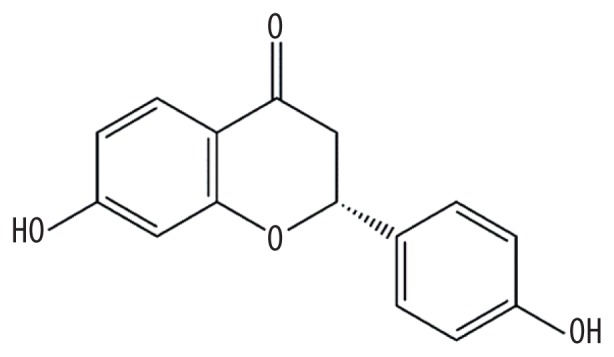
|
71.12 | 0.18 | Kushen |
| MOL005100 | 5,7-dihydroxy-2-(3-hydroxy-4-methoxyphenyl)chroman-4-one |
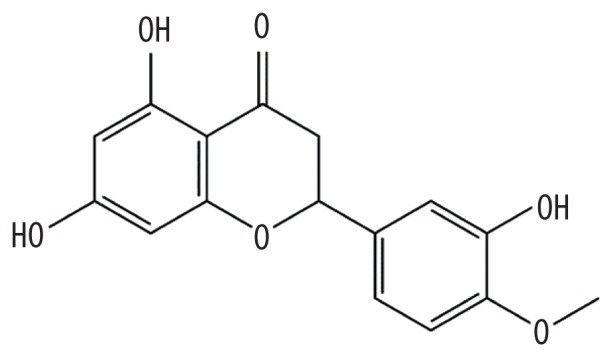
|
47.74 | 0.27 | Kushen |
| MOL005944 | Matrine |
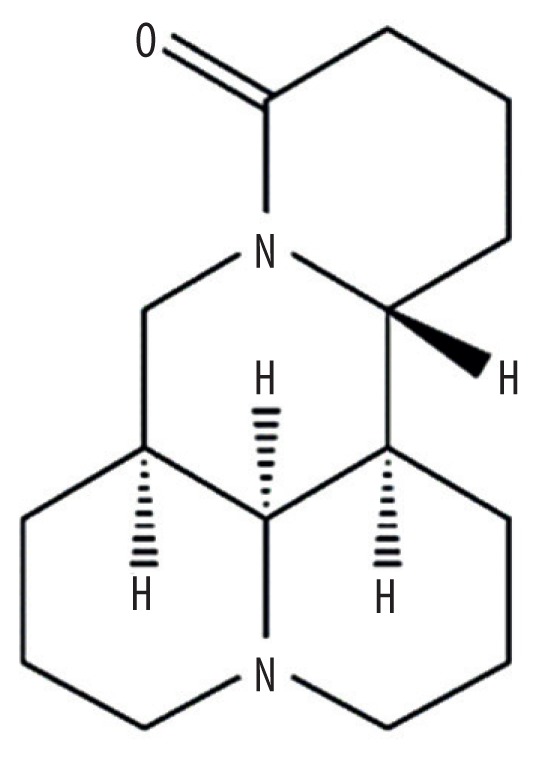
|
63.77 | 0.25 | Kushen |
| MOL000006 | Luteolin |
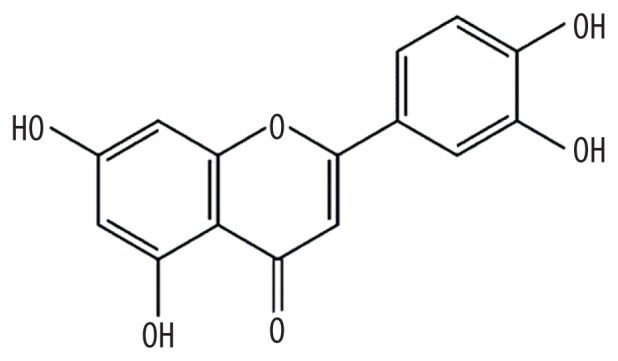
|
36.16 | 0.25 | Kushen |
| MOL006596 | Glyceollin |
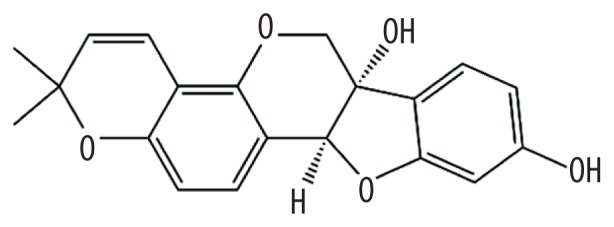
|
97.27 | 0.76 | Kushen |
| MOL003347 | Hyperforin |
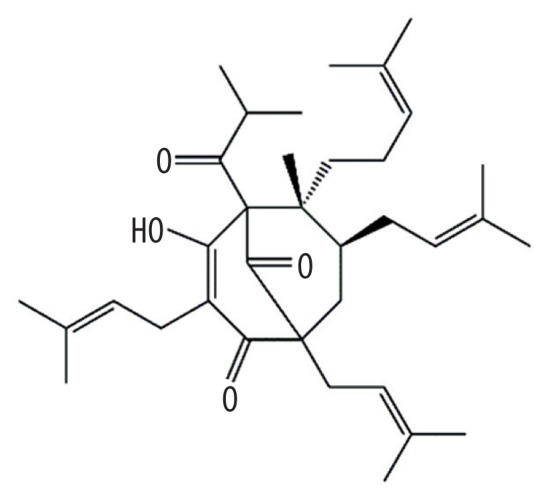
|
44.03 | 0.6 | Kushen |
| MOL006604 | (2S)-7-hydroxy-2-(4-hydroxyphenyl)-5-methoxy-8-(3-methylbut-2-enyl)chroman-4-one |
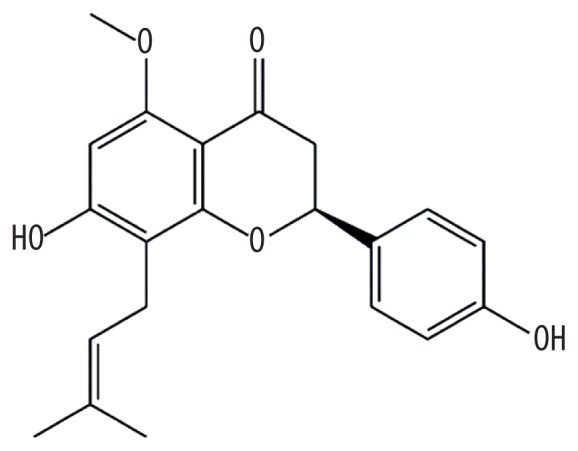
|
48.09 | 0.39 | Kushen |
| MOL006613 | Kushenin |
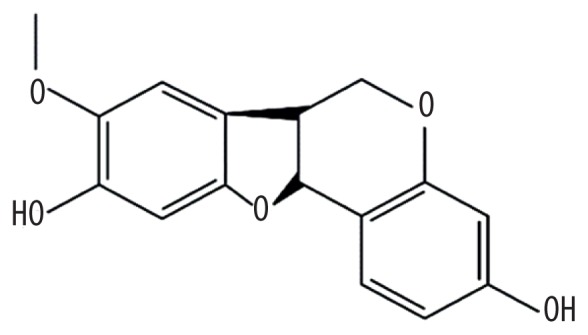
|
47.62 | 0.38 | Kushen |
| MOL006620 | Kushenol J_qt |
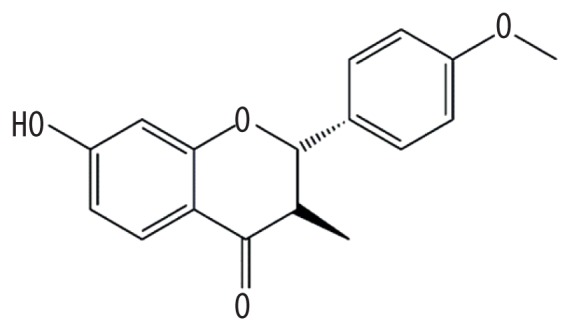
|
50.86 | 0.24 | Kushen |
| MOL006623 | Kushenol,t |
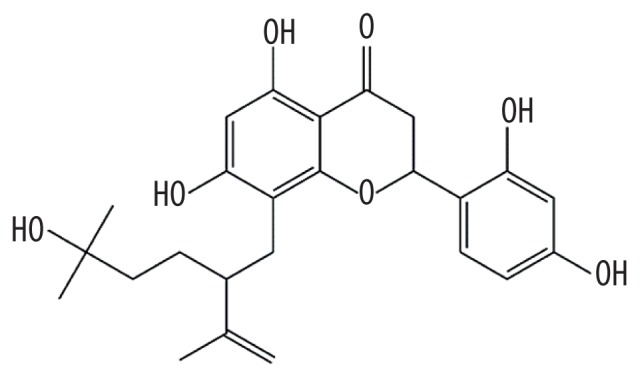
|
51.28 | 0.64 | Kushen |
| MOL006626 | Leachianone,g |
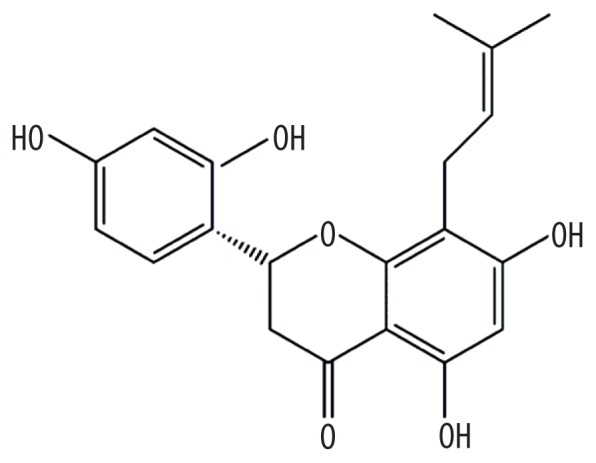
|
60.97 | 0.4 | Kushen |
| MOL006630 | Norartocarpetin |
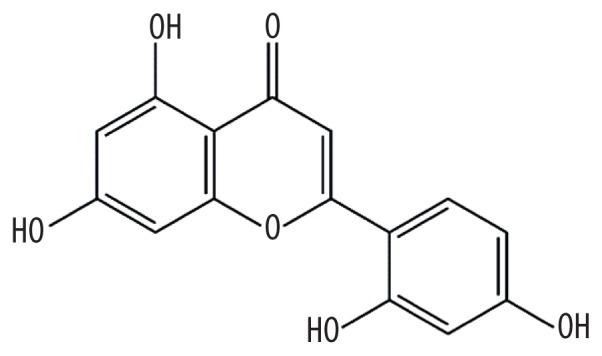
|
54.93 | 0.24 | Kushen |
| MOL000456 | Phaseolin |
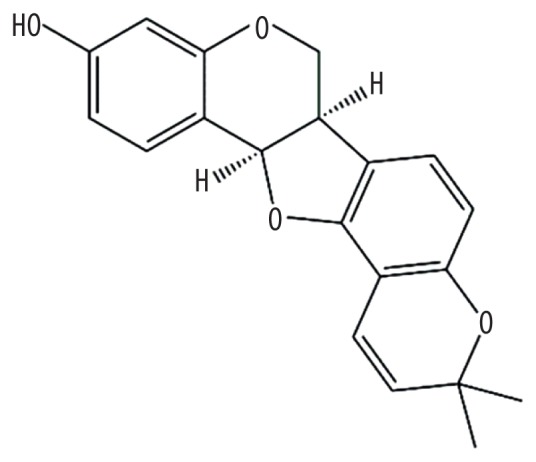
|
78.2 | 0.73 | Kushen |
| MOL000098 | Quercetin |
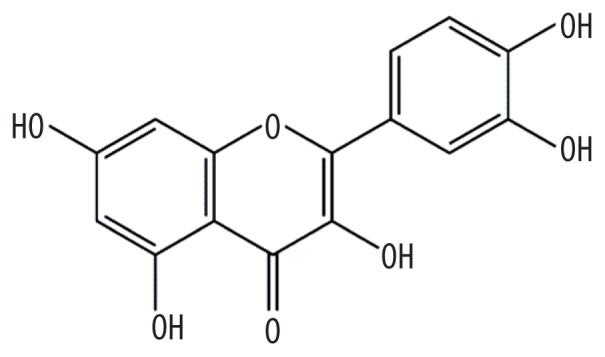
|
46.43 | 0.28 | Kushen or Baituling |
| MOL013117 | 4,7-dihydroxy-5-methoxyl-6-methyl-8-formyl-flavan |
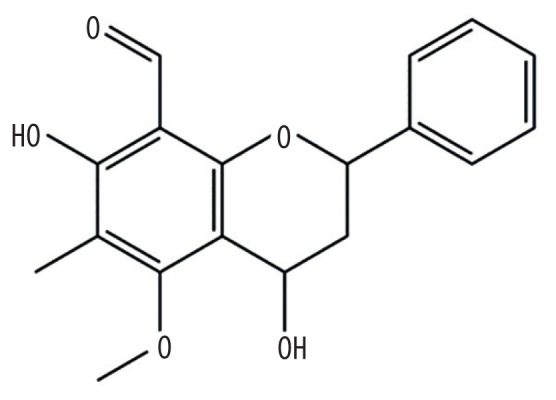
|
37.03 | 0.28 | Baituling |
| MOL013119 | Enhydrin |
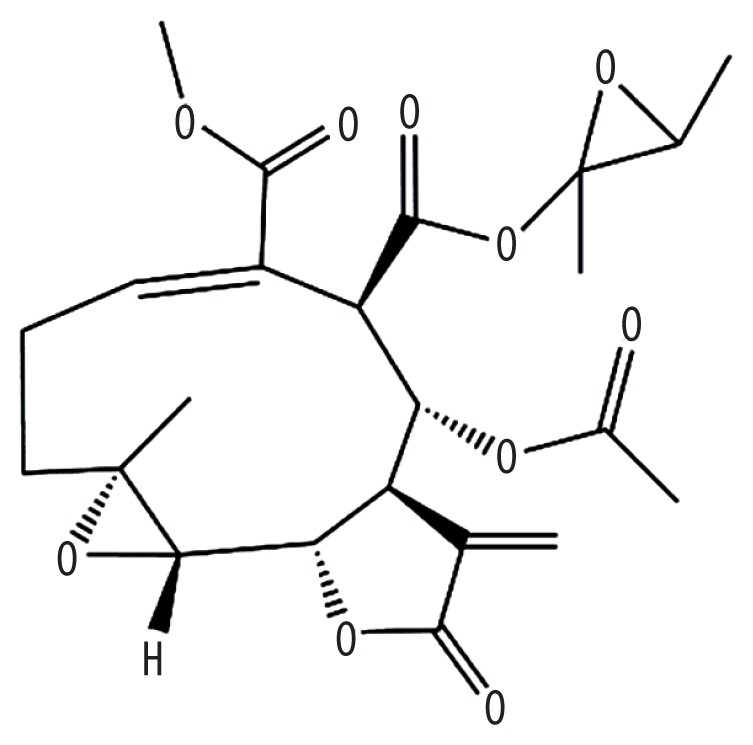
|
40.56 | 0.74 | Baituling |
| MOL013129 | (2R,3R)-2-(3,5-dihydroxyphenyl)-3,5,7-trihydroxychroman-4-one |
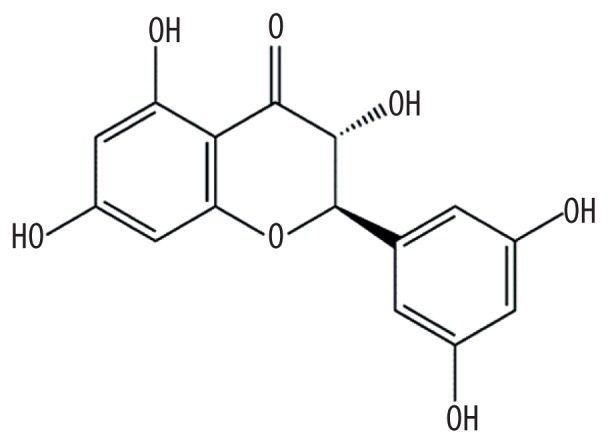
|
63.17 | 0.27 | Baituling |
| MOL001736 | (−)-Taxifolin |
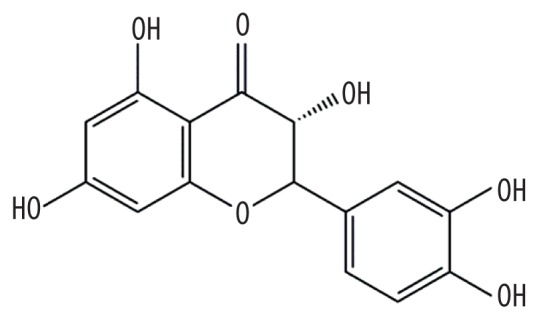
|
60.51 | 0.27 | Baituling |
| MOL000358 | beta-Sitosterol |
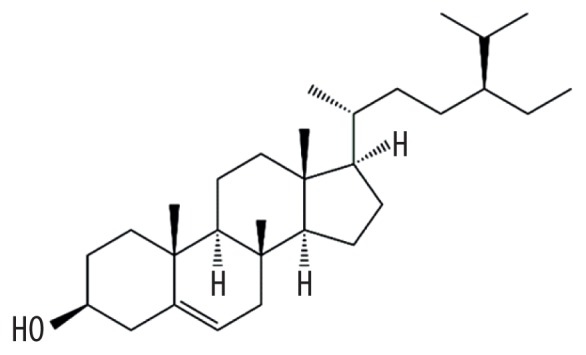
|
36.91 | 0.75 | Baituling |
| MOL000359 | Sitosterol |
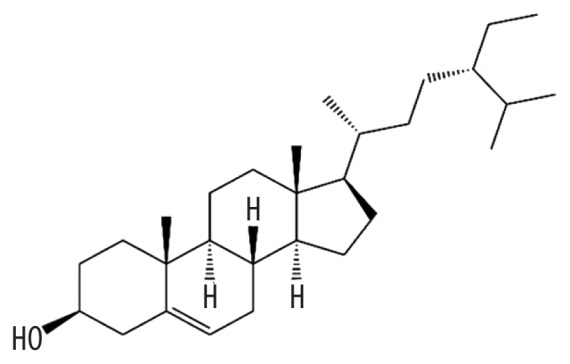
|
36.91 | 0.75 | Baituling |
| MOL004328 | Naringenin |
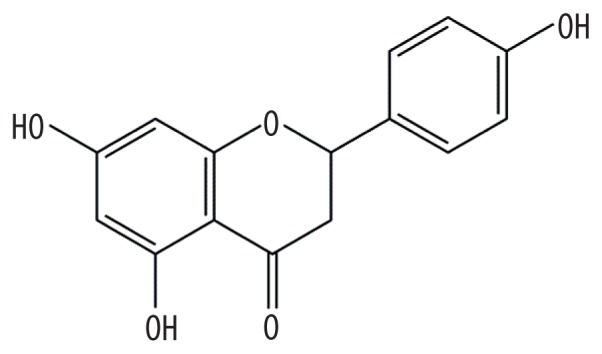
|
59.29 | 0.21 | Baituling |
| MOL000449 | Stigmasterol |
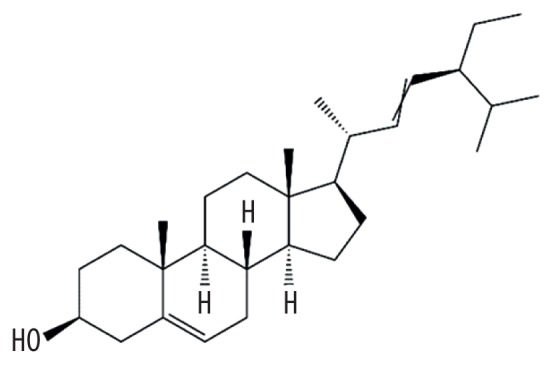
|
43.83 | 0.76 | Baituling |
| MOL004576 | Taxifolin |
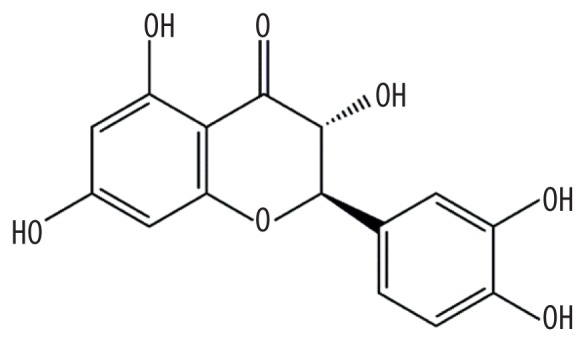
|
57.84 | 0.27 | Baituling |
| MOL000546 | Diosgenin |
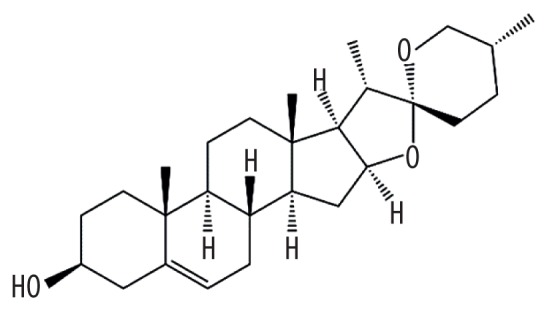
|
80.88 | 0.81 | Baituling |
Construction and analysis of drug-active ingredient-target gene network
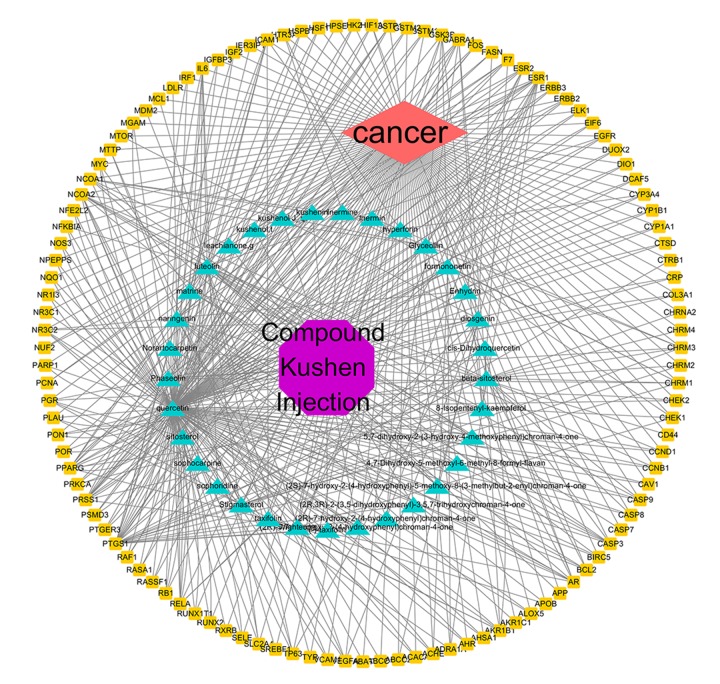
Thirty-three effective active ingredients, cancer, and 113 “drug-disease” key target genes were introduced into Cytoscape 3.7.1 software to construct a visualized active ingredient-cancer target network (Figure 2). There are 148 nodes and 494 lines shown in Figure 2; the octagonal purple node is the Chinese medicine preparation CKI; the red diamond node is cancer; the yellow oval node is 113 core target genes; the green triangle node represents 33 effective active ingredients. According to the analysis of the network, each effective active ingredient acts on at least 1 target gene, and each gene is regulated by at least 2 active ingredients, indicating the action characteristics of CKI multiple active components and multiple targets. The active components of CKI with the most target gene are quercetin, luteolin, naringenin, phaseolin, stigmasterol, formononetin, diosgenin, wighteone, etc. In addition, the main targets of the active ingredient and target gene linkage degree >10 are PTGS1, PRSS1, PPARG, NCOA2, and ESR1. This study speculates that these targets might be a key target for CKI treatment of cancer.
Figure 2.
Drug-component target network of active ingredients of compound Kushen injection (CKI).
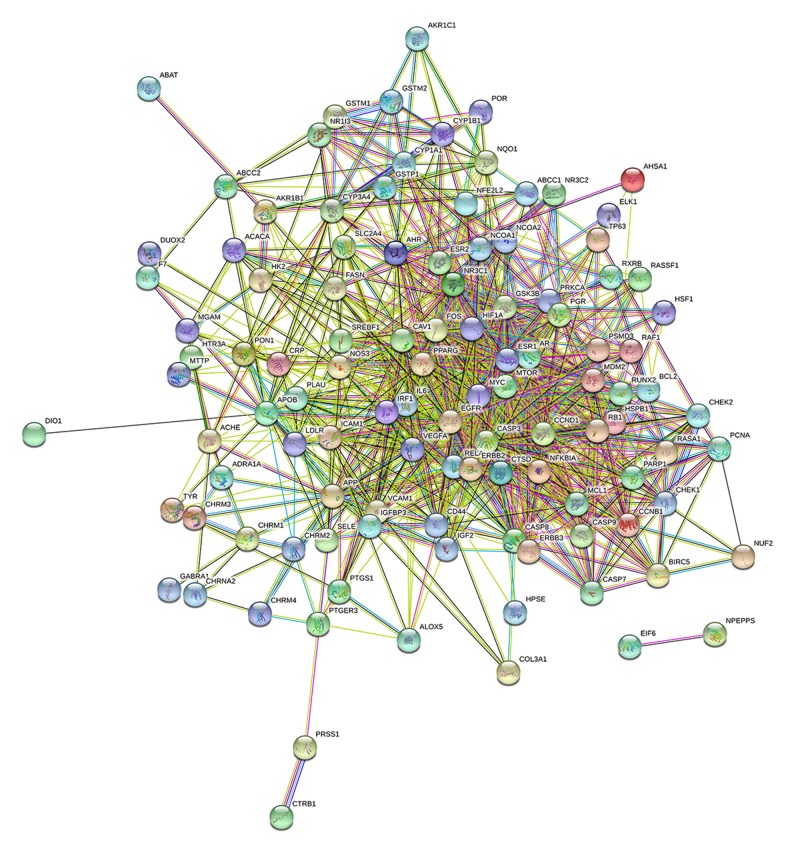
Analysis of target protein interaction network (PPI)
The STRING database platform was used to construct a network of target protein interactions, and the targets of CKI active components and cancer target genes were imported. The selected species were “Homo sapiens”, and the combined score >0.4. Threshold, the final protein-protein interaction network was obtained (Figure 3). As can be seen from Figure 3, there are a total of 113 solid circles of many colors, each circle represents a key target gene, and the center of the dot shows the protein structure of the target gene. In the PPI network, the linkage of each target gene represents a meaning, such as protein homology, gene co-expression, gene co-evolution, and inter-genetic adjacency. Statistical analysis was performed on each target gene to obtain the top 30 proteins with the highest number of adjacent genes (Figure 4). The proteins with more than 40 connectivity degrees were IL6, EGFR, CASP3, VEGFA, MYC, ESR1, CCND1, AR, ERBB2, FOS, MTOR, and PPARG. The more adjacent genes, the more prominent the role of this gene in the network, and further speculation may be the key targets for anti-cancer effects.
Figure 3.
Anti-cancer protein target interaction network (PPI).
Figure 4.
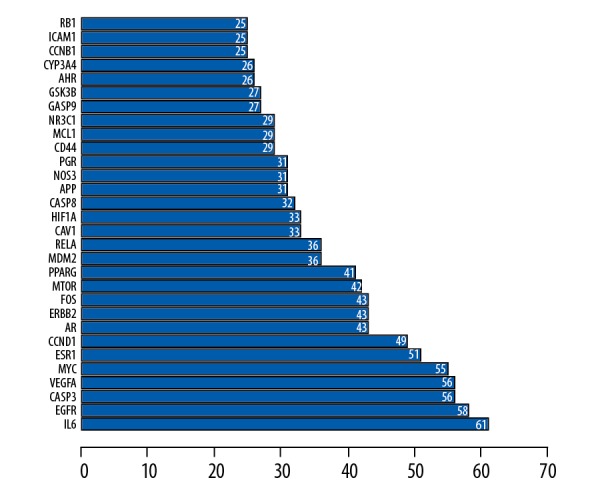
Protein interaction relationship histogram of compound Kushen injection (CKI).
GO biological function annotation and KEGG pathway enrichment analysis for targets
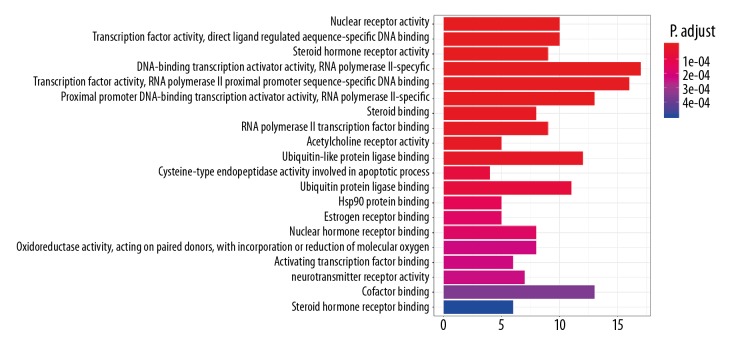
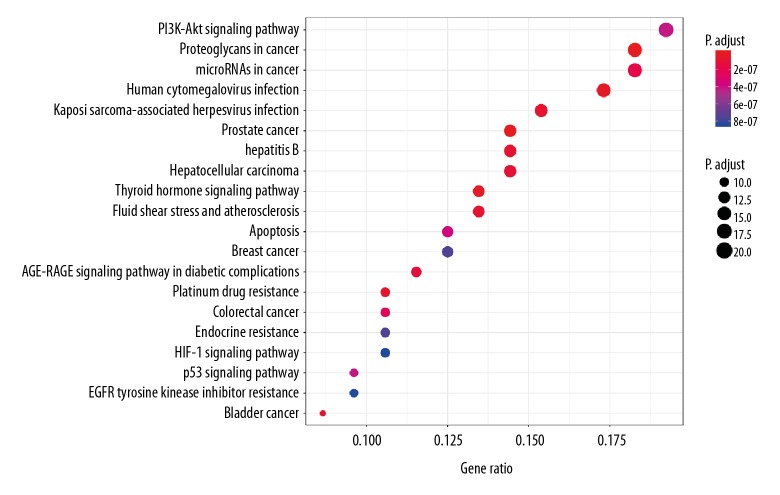
Then 113 target genes of the drug active ingredient-cancer intersection were introduced into Bioconductor bioinformatics data processing tools. Filtering was performed with P value=0.05 and Q value=0.05 as threshold values, and the GO biological function and KEGG signaling pathway were enriched and analyzed by R statistical programming language. There were 129 biological processes obtained through the enrichment of GO biological functions. We then selected the top 20 functional terms with high enrichment for analysis. The adjusted P value represents the significance of the biological process. The more the color of the column is red, the higher the enrichment. The results indicate that the targets mainly were associated with nuclear receptor activity, transcription factor activity, direct ligand regulated sequence-specific DNA binding, steroid hormone receptor activity, RNA polymerase II-specific, RNA polymerase II proximal promoter sequence-specific DNA binding, steroid binding, RNA polymerase II transcription factor binding, acetylcholine receptor activity, ubiquitin-like protein ligase binding, cysteine-type endopeptidase activity involved in apoptotic process, Hsp90 protein binding, estrogen receptor binding, and nuclear hormone receptor binding (Figure 5). Through the KEGG enrichment analysis, 93 related signaling pathways were obtained. The top 20 signal paths with high confidence and P-value less than or equal to 0.01 are selected for analysis as showed in Figure 6. It can be seen from Figure 6 that the majority of the target genes mainly affect substances or signaling pathways, including proteoglycans, thyroid hormone signaling pathway, human cytomegalovirus, Kaposi sarcoma-associated herpesvirus, AGE-RAGE signaling pathway, microRNAs, PI3K-Akt signaling pathway, p53 signaling pathway, HIF-1 signaling pathway. Furthermore, prostate cancer, bladder cancer, hepatitis B, hepatocellular carcinoma, colorectal cancer, breast cancer and so on were also high correlation to the key target genes. From these results, it is predicted that CKI might achieve anti-cancer effects mainly by regulating the aforementioned signal pathways or target genes associated with various cancers.
Figure 5.
Enrichment analysis of Gene Ontology (GO) biological process of anti-cancer targets gene from active ingredients of compound Kushen injection (CKI).
Figure 6.
Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway which related to anti-cancer for targets of compound Kushen injection (CKI).
Discussions
In modern medicine, cancer is caused by various carcinogenic factors, leading to abnormal cell proliferation and differentiation, which causes local tissues or organs to form lumps, also known as tumors. Benign tumors have the characteristics of clear borders, difficulty in metastasis and recurrence, no compression on tissues or organs, and can be surgically removed. However, malignant tumors grow faster, destroy the function of surrounding tissues or organs, the boundary is unclear and easy to metastasize, and it is easy to relapse after surgery, eventually causing the patient to die due to organ failure [20]. The classification of cancer is usually named after the cancerous tissue or organ, such as colon cancer, liver cancer, lung cancer, stomach cancer and breast cancer. At present, the main treatment of cancer by Western medicine is surgery-based, supplemented by chemotherapy, radiotherapy and molecular targeted drugs, but at the same time chemotherapy and radiotherapy have a serious negative impact on the normal cells of cancer patients [21,22]. In recent years, traditional Chinese medicine has had good effects in the treatment of cancer, including assisting against cancer, reducing adverse reactions after Western medicine treatment, effectively promoting the recovery of organ function, and controlling complications [23,24].
As a traditional Chinese medicine preparation, CKI can be used for clearing away heat and dampness, cooling blood and detoxifying and relieving cancer or tumor pain [25], which has been proven to have significant effects against cancer or tumor [26,27]. In recent years, due to the extensive research on natural drug products by experts and scholars in the Chinese medicine industry, it has been found that there are many components in Chinese herbal medicine, and different components play different pharmacological effects [28]. CKI were a mixture of natural compounds extracted from Kushen (Sophorae Flavescentis Radix) and Baituling (Smilacis Glabrae Rhixoma), which have been used for adjuvant anti-cancer clinical therapy for more than 15 years. The main components of the Chinese herbal medicine Kushen, matrine and oxymatrine, have a variety of pharmacological activities, including anti-inflammatory, anti-viral, anti-allergic, and cardiovascular protection, especially in anti-cancer, they have a potential effect [29]. Recently, various bioactivities of Smilacis Glabrae Rhixoma have been demonstrated by in vitro and in vivo assays, which include anti-inflammatory [30], antiviral [31], anti-cancer [32] and immunomodulatory effects [33].
The active ingredients-target gene network constructed in this study shows that 33 cancer-related active ingredients of CKI, such as quercetin, phaseolin, naringenin, matrine, luteolin, glyceollin, formononetin, diosgenin, stigmasterol, taxifolin, wighteone, 8-isopentenyl-kaempferol, and beta-sitosterol. Accumulation of relevant literature or data highlights that these 33 active ingredients are closely related to anti-cancer signaling pathways. Quercetin has been demonstrated to have diverse biological effects, including inhibition of cell proliferation in various cancers, as well as involvement in anti-cancer signal transduction pathways [34]. Naringenin causes apoptosis signal-regulation kinase 1 (ASK1) induced apoptosis mediated by reactive oxygen species (ROS), thereby revealing a mechanism for inhibiting pancreatic cancer cells [35]. Recent evidence demonstrated that luteolin directly regulates the potential inhibitory effects on colorectal cancer cells by modulating pleiotrophin (PTN) via miR-384 expression [36]. In one study [37], glyceollin was used in mouse mammary for in vivo/in vitro experiments. Glyceollin exert anti-proliferative effects mainly by reducing the expression of mitosis-related factors and encoding cell cycle. It is further demonstrated that glyceollin has the therapeutic potential for breast cancer. It was found that formononetin inhibits the proliferation of bladder cancer cells by modulating the miR-21-mediated PTEN/Akt signaling pathway [38].
Target protein interaction network (PPI) analysis found that the main target proteins of CKI active components and anti-cancer are IL6, EGFR, CASP3, VEGFA, MYC, ESR1, CCND1, AR, ERBB2, FOS, MTOR, and PPARG. Among them, IL6 is a pleiotropic cytokine, which induces epithelial-mesenchymal transition of cancer cells, thereby enhancing the invasiveness and distant metastasis ability of cancer cells and exhibits high expression in blood and tissues of cancer patients. A report showed that formononetin reduces IL6 expression by regulating mRNA [39]. EGFR is an epidermal growth factor receptor that over-expressed and induces proliferation in cancer. Luteolin induces apoptosis in glioblastoma through a downstream signal mediated Akt/MAPK signaling pathway activated by EGFR [40]. Vascular endothelial growth factor (VEGF) is mainly involved in neovascularization, which can upregulate the expression of many known tumors, and its expression is associated with tumor stage and progression. One study reported that low concentrations of quercetin might inhibit the migration and angiogenesis of cancer cells by downregulating VEGF protein levels [41]. ESR1 is an estrogen receptor that binds to estrogen in the body to promote the proliferation and differentiation of breast cells and is also involved in the pathological processes of breast cancer, endometrial cancer or osteoporosis [42]. AR is an androgen receptor, which is highly expressed in tissues such as prostate, testis, ovary and fat. It is closely related to the development of various types of cancer such as prostate cancer, bladder cancer or liver cancer.
The GO biological function and KEGG pathway suggested these targets are mainly associated with signal related pathways, such as thyroid hormone signaling pathway, AGE-RAGE signaling pathway, PI3K-Akt signaling pathway, p53 signaling pathway and HIF-1 signaling pathway. The main role of thyroid hormone is to promote the metabolism of matter and energy, maintain the body’s growth and development, and at the same time regulates angiogenesis. Natural thyroid hormone analogs can affect tumor-associated angiogenesis and are important implications for cancer treatment [43]. One study revealed that advanced glycation end products and their receptors (AGE-RAGE) interactions are associated with prostate cancer and may be enhanced prostate cancer cell proliferation by the PI3K/Akt signaling pathway [44]. Phosphoinositide 3-kinase (PI3K) signaling is involved in the regulation of cell functions such as proliferation, differentiation, apoptosis, and glucose transport. The PI3K-Akt signaling pathway is closely related to cancer proliferation, invasion, and metastasis and is one of the most frequent and critical signaling pathways in cancer. The active ingredient naringenin in CKI inhibits proliferation and induces apoptosis of prostate cancer cells through the PI3K/AKT signaling pathway [45]. Matrine is the active ingredient that also inhibits the proliferation and metastasis of gastric cancer cells through the PI3K/AKT pathway [46]. Functional genomics studies reveal that p53 is a tumor suppressor gene in vivo that is involved in the expression of multiple genes involved in apoptosis, growth arrest or senescence, and the p53 pathway is one of the crucial signaling pathways for cancer cell apoptosis. The active ingredient luteolin can be a molecule for the therapy of cancer by acting on p53 signaling pathway [47]. Hypoxia-inducible factor 1 alpha (HIF-1α) is a regulator that plays an important role in tumor cell growth and proliferation, which the mechanism of action is related to oxygen transport and angiogenesis. Downregulation of HIF-1α can inhibit the proliferation, migration, and invasion of cancer [48]. Therefore, we found that these signaling pathways are closely related to cancer, and the active components in CKI can exert anti-cancer effects through these signaling pathways.
Conclusions
CKI as traditional Chinese medicine injection widely used in clinical practice, might contain many components related to pharmacological activity. It is still unclear which components exert anti-cancer effects or mechanisms of action. In this study, bioinformatics and network pharmacology were applied to explore the mechanism of CKI multi-component multi-targeting to treat cancer. The results of this study indicate that we have determined that CKI is rich in 33 anti-cancer related active ingredients, and some of its active anti-cancer effects have been confirmed by some reports, such as quercetin, luteolin, naringenin, phaseolin, stigmasterol, formononetin, diosgenin and wighteone. In the enrichment analysis of KEGG, PI3K-Akt and p53 signaling pathways are important anti-cancer pathways for CKI active components, and the active ingredients of CKI have a certain therapeutic effect on prostate cancer, bladder cancer, hepatocellular carcinoma, colorectal cancer, and breast cancer. The comprehensive data of this study can provide a theoretical basis for screening promising cancer drug candidates, but still require in vivo or in vitro experimental verification of CKI active ingredients.
Footnotes
Source of support: Departmental sources
Conflict of interest
None.
References
- 1.Arruebo M, Vilaboa N, Saez-Gutierrez B, et al. Assessment of the evolution of cancer treatment therapies. Cancers (Basel) 2011;3(3):3279–330. doi: 10.3390/cancers3033279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang W, You RL, Qin WJ, et al. Anti-tumor activities of active ingredients in compound Kushen injection. Acta Pharmacol Sin. 2015;36(6):676–79. doi: 10.1038/aps.2015.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ma Y, Gao H, Liu J, et al. Identification and determination of the chemical constituents in a herbal preparation, compound Kushen injection, by HPLC and LC-DAD-MS/MS. Journal of Liquid Chromatography. 2014;37(2):207–20. [Google Scholar]
- 4.Hao G, Zheng J, Huo R, et al. Smilax glabra Roxb targets Akt (p-Thr308) and inhibits Akt-mediated signaling pathways in SGC7901 cells. J Drug Target. 2016;24(6):557–65. doi: 10.3109/1061186X.2015.1113540. [DOI] [PubMed] [Google Scholar]
- 5.Wu CW, Lu L, Liang SW, et al. [Application of drug-target prediction technology in network pharmacology of traditional Chinese medicine]. Zhongguo Zhong Yao Za Zhi. 2016;1(3):377–82. doi: 10.4268/cjcmm20160303. [DOI] [PubMed] [Google Scholar]
- 6.Shao L, Zhang B. Traditional Chinese medicine network pharmacology: Theory, methodology and application. Chin J Nat Med. 2013;11(2):110–20. doi: 10.1016/S1875-5364(13)60037-0. [DOI] [PubMed] [Google Scholar]
- 7.Hsiao WL, Liu L. The role of traditional Chinese herbal medicines in cancer therapy – from TCM theory to mechanistic insights. Planta Med. 2010;76(11):1118–31. doi: 10.1055/s-0030-1250186. [DOI] [PubMed] [Google Scholar]
- 8.Liu H, Wang J, Zhou W, et al. Systems approaches and polypharmacology for drug discovery from herbal medicines: An example using licorice. J Ethnopharmacol. 2013;146(3):773–93. doi: 10.1016/j.jep.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 9.Wang S, Wang H, Lu Y, et al. Tianfoshen oral liquid: A CFDA approved clinical traditional Chinese medicine, normalizes major cellular pathways disordered during colorectal carcinogenesis. Oncotarget. 2017;8(9):14549–69. doi: 10.18632/oncotarget.14675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang YQ, Xia M, Guo QY, et al. Network pharmacology-based approaches capture essence of Chinese herbal medicines. Chinese Herbal Medicines. 2016;8(2):107–116. [Google Scholar]
- 11.Xu X, Zhang W, Huang C, et al. A novel chemometric method for the prediction of human oral bioavailability. Int J Mol Sci. 2012;13(6):6964–6982. doi: 10.3390/ijms13066964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chandran U, Patwardhan B. Network ethnopharmacological evaluation of the immunomodulatory activity of Withania somnifera. J Ethnopharmacol. 2017;197:250–256. doi: 10.1016/j.jep.2016.07.080. [DOI] [PubMed] [Google Scholar]
- 13.Chen CY, Kao CL, Liu CM. The cancer prevention, anti-inflammatory and anti-oxidation of bioactive phytochemicals targeting the TLR4 signaling pathway. Int J Mol Sci. 2018;19(9) doi: 10.3390/ijms19092729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ong CS, Zhou J, Ong CN, et al. Luteolin induces G1 arrest in human nasopharyngeal carcinoma cells via the Akt-GSK-3β-cyclin D1 pathway. Cancer Lett. 2010;298(2):167–175. doi: 10.1016/j.canlet.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 15.Rajavel T, Packiyaraj P, Suryanarayanan V, et al. β-Sitosterol targets Trx/Trx1 reductase to induce apoptosis in A549 cells via ROS mediated mitochondrial dysregulation and p53 activation. Sci Rep. 2018;8(1):2071. doi: 10.1038/s41598-018-20311-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu LH, Lin C, Lin HY, et al. Naringenin suppresses neuroinflammatory responses through inducing suppressor of cytokine signaling 3 expression. Mol Neurobiol. 2016;53(2):1080–91. doi: 10.1007/s12035-014-9042-9. [DOI] [PubMed] [Google Scholar]
- 17.Watjen W, Kulawik A, Suckow-Schnitker AK, et al. Pterocarpans phaseollin and neorautenol isolated from Erythrina addisoniae induce apoptotic cell death accompanied by inhibition of ERK phosphorylation. Toxicology. 2007;242(1–3):71–79. doi: 10.1016/j.tox.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 18.Kim YS, Li XF, Kang KH, et al. Stigmasterol isolated from marine microalgae Navicula incerta induces apoptosis in human hepatoma HepG2 cells. BMB Rep. 2014;47(8):433–38. doi: 10.5483/BMBRep.2014.47.8.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu XY, Xu H, Wu ZF, et al. Formononetin, a novel FGFR2 inhibitor, potently inhibits angiogenesis and tumor growth in preclinical models. Oncotarget. 2015;6(42):44563–78. doi: 10.18632/oncotarget.6310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang YL, Liu L, Wang Y, et al. The prevalence of depression and anxiety among Chinese adults with cancer: A systematic review and meta-analysis. BMC Cancer. 2013;13:393. doi: 10.1186/1471-2407-13-393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hasan MR, Bari MA, Alam S, et al. Concurrent weekly versus three weekly cisplatin with radiotherapy in locally advanced uterine cervical carcinoma. JNMA J Nepal Med Assoc. 2018;56(213):842–47. doi: 10.31729/jnma.3636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goswami R, Subramanian G, Silayeva L, et al. Gene therapy leaves a vicious cycle. Front Oncol. 2019;9:297. doi: 10.3389/fonc.2019.00297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liao YH, Li CI, Lin CC, et al. Traditional Chinese medicine as adjunctive therapy improves the long-term survival of lung cancer patients. J Cancer Res Clin Oncol. 2017;143(12):2425–35. doi: 10.1007/s00432-017-2491-6. [DOI] [PubMed] [Google Scholar]
- 24.Lin J, Chen L, Qiu X, et al. Traditional Chinese medicine for human papillomavirus (HPV) infections: A systematic review. Biosci Trends. 2017;11(3):267–73. doi: 10.5582/bst.2017.01056. [DOI] [PubMed] [Google Scholar]
- 25.Guo Y, Huang Y, Shen H, et al. Efficacy of compound Kushen injection in relieving cancer-related pain: A systematic review and meta-analysis. Evid Based Complement Alternat Med. 2015;2015 doi: 10.1155/2015/840742. 840742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ao M, Xiao X, Li Q, et al. Efficacy and safety of compound Kushen injection combined with chemotherapy on postoperative Patients with breast cancer: A meta-analysis of randomized controlled trials. Medicine (Baltimore) 2019;98(3):e14024. doi: 10.1097/MD.0000000000014024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yu L, Zhou Y, Yang Y, et al. Efficacy and safety of compound Kushen injection on patients with advanced colon cancer: A meta-analysis of randomized controlled trials. Evid Based Complement Alternat Med. 2017;2017 doi: 10.1155/2017/7102514. 7102514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang CY, Bai XY, Wang CH, et al. Traditional Chinese medicine: A treasured natural resource of anticancer drug research and development. Am J Chin Med. 2014;42(3):543–59. doi: 10.1142/S0192415X14500359. [DOI] [PubMed] [Google Scholar]
- 29.Liu Y, Xu Y, Ji W, et al. Anti-tumor activities of matrine and oxymatrine: Literature review. Tumour Biol. 2014;35(6):5111–19. doi: 10.1007/s13277-014-1680-z. [DOI] [PubMed] [Google Scholar]
- 30.Galhena PB, Samarakoon SR, Thabrew MI, et al. Anti-inflammatory activity is a possible mechanism by which the polyherbal formulation comprised of Nigella sativa (seeds), Hemidesmus indicus (root), and Smilax glabra (rhizome) mediates its antihepatocarcinogenic effects. Evid Based Complement Alternat Med. 2012;2012 doi: 10.1155/2012/108626. 108626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ooi LS, Sun SS, Wang H, et al. New mannose-binding lectin isolated from the rhizome of Sarsaparilla Smilax glabra Roxb. (Liliaceae) J Agric Food Chem. 2004;52(20):6091–95. doi: 10.1021/jf030837o. [DOI] [PubMed] [Google Scholar]
- 32.Samarakoon SR, Thabrew I, Galhena PB, et al. Modulation of apoptosis in human hepatocellular carcinoma (HepG2 cells) by a standardized herbal decoction of Nigella sativa seeds, Hemidesmus indicus roots and Smilax glabra rhizomes with anti- hepatocarcinogenic effects. BMC Complement Alternat Med. 2012;12:25. doi: 10.1186/1472-6882-12-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jiang J, Xu Q. Immunomodulatory activity of the aqueous extract from rhizome of Smilax glabra in the later phase of adjuvant-induced arthritis in rats. J Ethnopharmacol. 2003;85(1):53–59. doi: 10.1016/s0378-8741(02)00340-9. [DOI] [PubMed] [Google Scholar]
- 34.Brito AF, Ribeiro M, Abrantes AM, et al. Quercetin in cancer treatment, alone or in combination with conventional therapeutics? Curr Med Chem. 2015;22(26):3025–39. doi: 10.2174/0929867322666150812145435. [DOI] [PubMed] [Google Scholar]
- 35.Park HJ, Choi YJ, Lee JH, et al. Naringenin causes ASK1-induced apoptosis via reactive oxygen species in human pancreatic cancer cells. Food Chem Toxicol. 2017;99:1–8. doi: 10.1016/j.fct.2016.11.008. [DOI] [PubMed] [Google Scholar]
- 36.Yao Y, Rao C, Zheng G, et al. Luteolin suppresses colorectal cancer cell metastasis via regulation of the miR384/pleiotrophin axis. Oncol Rep. 2019;42(1):131–41. doi: 10.3892/or.2019.7136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lecomte S, Chalmel F, Ferriere F, et al. Glyceollins trigger anti-proliferative effects through estradiol-dependent and independent pathways in breast cancer cells. Cell Commun Signal. 2017;15(1):26. doi: 10.1186/s12964-017-0182-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu Y, Zhang X, Li Z, et al. Formononetin inhibits human bladder cancer cell proliferation and invasiveness via regulation of miR-21 and PTEN. Food Funct. 2017;8(3):1061–66. doi: 10.1039/c6fo01535b. [DOI] [PubMed] [Google Scholar]
- 39.Li Z, Zeng G, Zheng X, et al. Neuroprotective effect of formononetin against TBI in rats via suppressing inflammatory reaction in cortical neurons. Biomed Pharmacother. 2018;106:349–54. doi: 10.1016/j.biopha.2018.06.041. [DOI] [PubMed] [Google Scholar]
- 40.Anson DM, Wilcox RM, Huseman ED, et al. Luteolin decreases epidermal growth factor receptor-mediated cell proliferation and induces apoptosis in glioblastoma cell lines. Basic Clin Pharmacol Toxicol. 2018;123(6):678–86. doi: 10.1111/bcpt.13077. [DOI] [PubMed] [Google Scholar]
- 41.Liu Y, Tang ZG, Yang JQ, et al. Low concentration of quercetin antagonizes the invasion and angiogenesis of human glioblastoma U251 cells. Onco Targets Ther. 2017;10:4023–28. doi: 10.2147/OTT.S136821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bartels S, Christgen M, Luft A, et al. Estrogen receptor (ESR1) mutation in bone metastases from breast cancer. Mod Pathol. 2018;31(1):56–61. doi: 10.1038/modpathol.2017.95. [DOI] [PubMed] [Google Scholar]
- 43.Schmohl KA, Nelson PJ, Spitzweg C, et al. Tetrac as an anti-angiogenic agent in cancer. Endocr Relat Cancer. 2019;26(6):287–304. doi: 10.1530/ERC-19-0058. [DOI] [PubMed] [Google Scholar]
- 44.Bao JM, He MY, Liu YW, et al. AGE/RAGE/Akt pathway contributes to prostate cancer cell proliferation by promoting Rb phosphorylation and degradation. Am J Cancer Res. 2015;5(5):1741–50. [PMC free article] [PubMed] [Google Scholar]
- 45.Lim W, Park S, Bazer FW, et al. Naringenin-induced apoptotic cell death in prostate cancer cells is mediated via the PI3K/AKT and MAPK signaling pathways. J Cell Biochem. 2017;118(5):1118–31. doi: 10.1002/jcb.25729. [DOI] [PubMed] [Google Scholar]
- 46.Peng X, Zhou D, Wang X, et al. Matrine suppresses proliferation and invasion of SGC7901 cells through inactivation of PI3K/Akt/uPA pathway. Ann Clin Lab Sci. 2016;46(5):457–62. [PubMed] [Google Scholar]
- 47.Ambasta RK, Gupta R, Kumar D, et al. Can luteolin be a therapeutic molecule for both colon cancer and diabetes? Brief Funct Genomics. 2018;18(4):230–39. doi: 10.1093/bfgp/ely036. [DOI] [PubMed] [Google Scholar]
- 48.Zhang J, Xu J, Dong Y, et al. Down-regulation of HIF-1alpha inhibits the proliferation, migration, and invasion of gastric cancer by inhibiting PI3K/AKT pathway and VEGF expression. Biosci Rep. 2018;38(6) doi: 10.1042/BSR20180741. pii: BSR20180741. [DOI] [PMC free article] [PubMed] [Google Scholar]