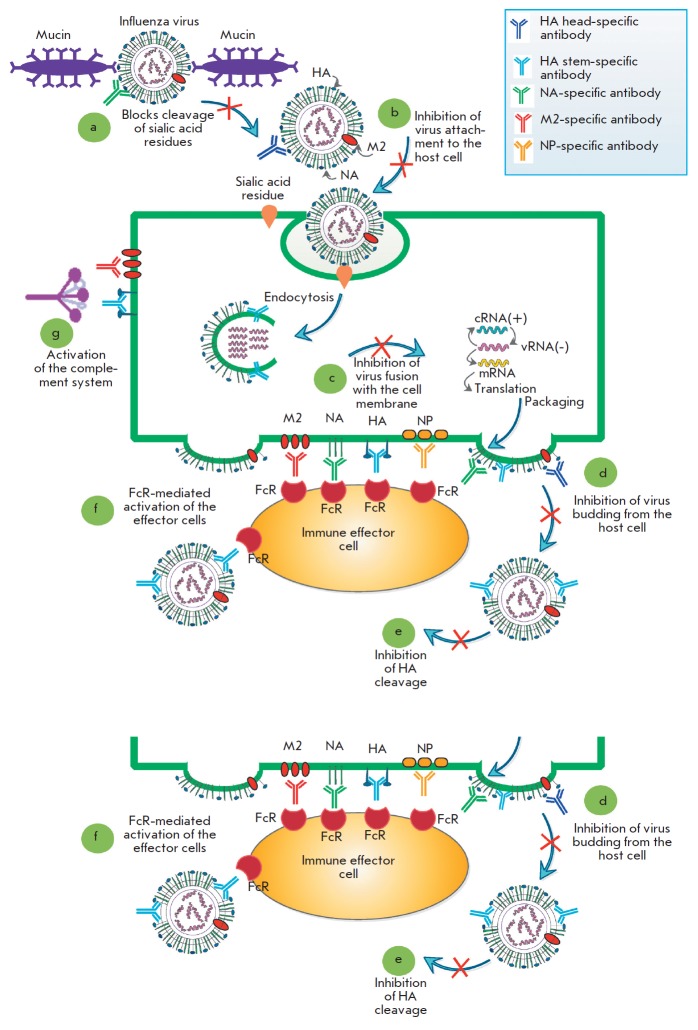
Fig. 1.
Mechanisms of action of anti-influenza antibodies. The influenza virus enters the body through respiratory tract mucosa, where viral hemagglutinin (HA) binds to the terminal sialic acids of mucin. Neuraminidase (NA) releases the virus by cleaving the terminal sialic acid residues. Antibodies to neuraminidase can inhibit the reaction, and the virus would not be able to penetrate the mucous layer (a). After penetrating the mucous layer, the influenza virus binds to the sialic acids on the surface of the target cells and enters the cell by endocytosis. Neutralizing antibodies bind to influenza HA and block this process (b).The endosomes of the target cells become acidified, thus triggering the fusion of the endosomal and viral membranes via HA, which results in the release of the viral genome into the cell cytoplasm. Antibodies to the stem domain of HA can inhibit this process (c).After the synthesis of viral proteins, the internal proteins are packed into viral particles containing HA, NA, and the M2 ion channel molecules on the virion surface. On the cell surface, the HA, NA, and M2 proteins can be bound by antibodies that block the budding of viral particles. Maturing viral particles are covered by the host cell membrane as a result of the interaction between HA and sialic acids. Meanwhile, NA cleaves terminal sialic acids from the virus, while antibodies to NA can inhibit this process (d). Finally, in the matured viral particles, HA0 is cleaved into the HA1 and HA2 subunits by the host proteases that are present in the respiratory tract. Antibodies directed to the HA stem domain can block this process (e).In addition, viral antigens exposed to the surface of an infected cell (including the internal protein NP, which is detected on the surface of the infected cell) are targets for antibodies that activate effector cells via the Fc-FcR interaction (f).Antibodies directed to the viral antigens exposed on the cell surface can also activate the complement system (g)