Figure 8.
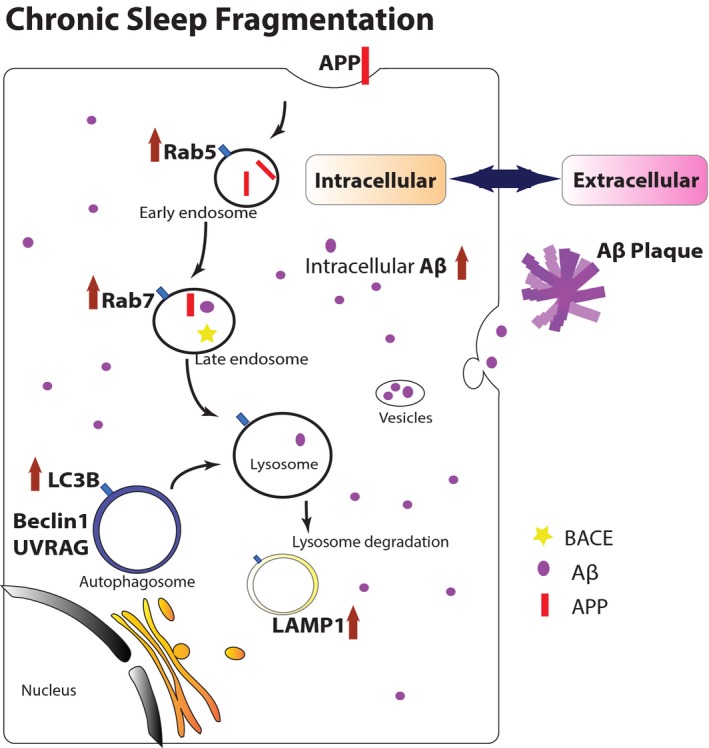
Schematic model of how CSF induced accumulation of endosomes, autophagosomes, and lysosomes, which led to increased intracellular Aβ accumulation. In normal conditions, the trafficking, catabolism, and elimination of APP are via the EAL pathway. First, APP is internalized into cells by endocytosis, then sorted in the early endosomes, and delivered to the late endosomes. APP can be processed by APP secretases to form Aβ in this stage. Then, the late endosomes fuse with either lysosomes or autophagosomes for lysosomal degradation. The balance of formation and clearance of Aβ is maintained by normal EAL pathway. After 2‐mo CSF, the EAL pathway is dysregulated and autophagic flux flow is impaired, leading to accumulation of abnormal vesicles in cells. It is evident by increased expression of Rab5 (early endosome marker), Rab7 (late endosome marker), Lamp1 (lysosome marker), as well as LC3B (autophagosome marker), and autophagy‐positive regulatory factors Beclin 1 and UVRAG. The accelerated APP processing in EAL pathway results in imbalance of formation and clearance of intracellular Aβ. In pathogenic process of Alzheimer's disease, overloaded intracellular Aβ can be released to extracellular space, potentially forming Aβ plaques eventually via oligomer polyermerization
