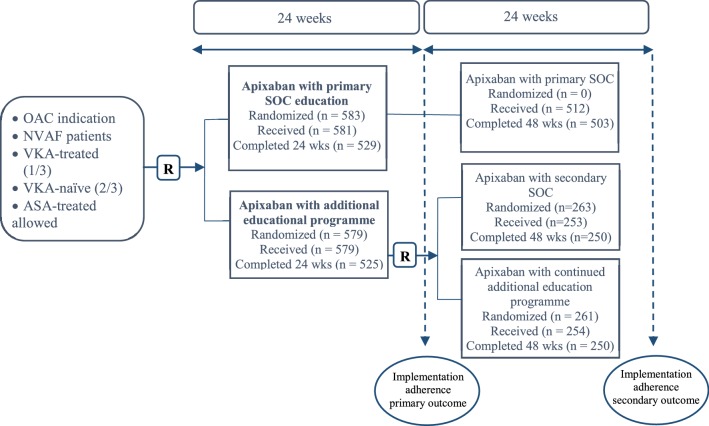
Fig. 1.
Study design. Patients consented to and initiated therapy with apixaban and were subsequently asked to participate in the implementation phase of the study. Following consent, patients were centrally randomized in a 1:1 ratio to receive standard-of-care patient information or an additional educational program. For subject discontinuations see Table 3 in the Electronic Supplementary Material. ASA acetylsalicylic acid, NVAF non-valvular atrial fibrillation, OAC oral anticoagulant, R randomization, SOC standard of care, VKA vitamin-K antagonist