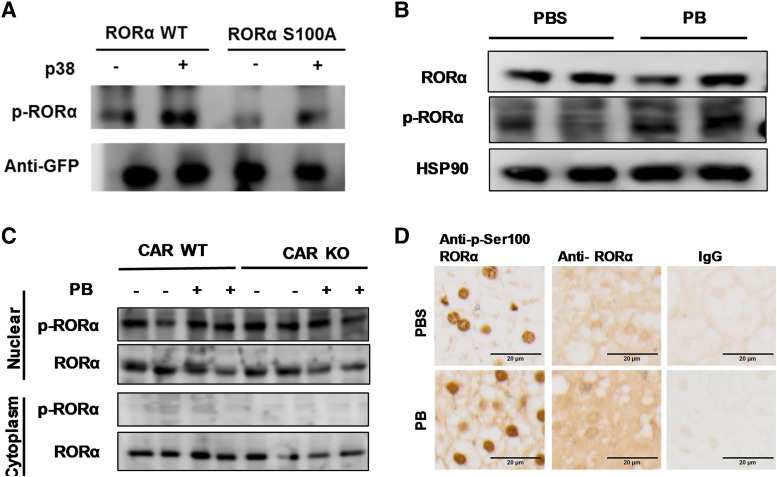
Fig. 5.
p-Ser100 RORα exists in nucleus in mouse liver. (A) RORα phosphorylation by p38 MAP kinase in HepG2 cell. HepG2 cells were transfected with RORα WT or RORα S100A with p38 MAP kinase expression vectors. Western blot assay was employed to determine phosphorylation of RORα S100 residue. (B) Expression of RORα and p-Ser100 RORα in human primary hepatocytes. The cells were treated with PBS or PB for 24 hours and the Western blot assay was determined in whole-cell lysate by anti-RORα or p-Ser100 RORα antibodies. HSP90, heat shock protein 90. (C and D) CAR WT and CAR KO mice treated with PB for 24 hours, and livers were collected for Western blot (C) or immunohistochemic staining (D) with anti-RORα or anti–p-Ser-RORα antibodies. Both immunohistochemic staining and Western blot assay findings suggested that though RORα was expressed both in the cytoplasm and nuclear fractions of the mouse liver, p-Ser100 RORα expression was limited in the nucleus.