Figure 3.
FAMIN Activities Are Evolutionarily Conserved and Adenosine Phosphorolysis Compromised in I254V
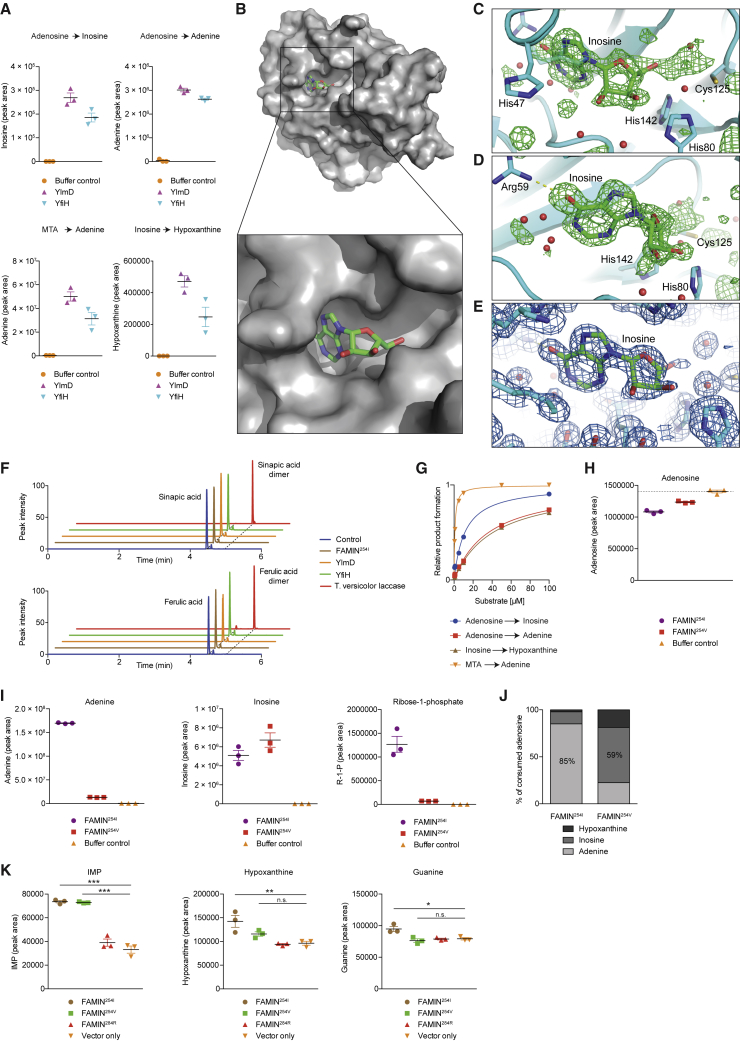
(A) Enzyme activities of YlmD and YfiH as measured by inosine, adenine, and hypoxanthine production (n = 3).
(B) Crystal structure of YlmD determined in the presence of inosine and phosphate, shown in molecular surface representation with a bound inosine as ball-and-stick.
(C and D) Substrate binding site of YlmD, polder Fo-Fc electron density map calculated at 1.5-Å resolution with inosine and bulk solvent omitted. Maps contoured at +3.5 σ (green mesh). (C) Cys125-His80-His142 located near inosine’s ribose moiety. The His47 side chain inserted into the purine-binding pocket in apo-YlmD (semi-transparent representation). (D) View rotated 45° around the y axis. The hypoxanthine moiety forms a hydrogen bond with the Arg59 side chain (dashed line). Selected ordered water molecules (red spheres).
(E) 2Fo-Fc electron density map near the bound inosine calculated after refinement with diffraction data to 1.2-Å resolution and contoured at +1.2 σ (blue mesh). Viewing orientation between those in (C) and (D).
(F) Representative extracted chromatograms demonstrating oxidation of laccase substrates sinapic and ferulic acid into dimer products after incubation with YlmD, YfiH, Strep-tagged FAMIN254I, laccase from Trametes versicolor, or appropriate control.
(G) Michaelis-Menten kinetics of FAMIN activities for indicated substrates.
(H and I) Consumption of adenosine (H) and production of adenine, inosine, and R1P (I) following incubation of Strep-tagged FAMIN254I, FAMIN254V, or buffer control with 100 μM adenosine (n = 3).
(J) Fractional conversion of adenosine into adenine versus inosine versus hypoxanthine following incubation of adenosine with Strep-tagged FAMIN254I or FAMIN254V (n = 3, mean).
(K) Inosine monophosphate (IMP), hypoxanthine, and guanine levels in HEK293T cells after transient transfection with FAMIN expression vectors or empty vector (n = 3).
Data represented as mean ± SEM. ∗p < 0.05, ∗∗p < 0.01, and ∗∗∗p < 0.001 (unpaired, two-tailed Student’s t test).