Abstract
Background and Objectives:
Device closure of secundum atrial septal defect is shown to be feasible and effective in children weighing ≤10 kg. Issues such as how large is too large, how to choose device size, does the length of the interatrial septum (IAS) matter, and need for technical modifications for successful device delivery have not been systematically addressed.
Materials and Methods:
This is a retrospective study, comprising 45 patients weighing ≤10 kg, who were chosen for device closure between January 2010 and June 2018. Patient selection was done on basis of transthoracic echocardiography. Device closure was done using Amplatzer septal occluder. The device size was selected primarily based on transesophageal echocardiography (TEE)-measured defect diameter. Although IAS length was taken into consideration, adequate rim size was the key factor in deciding device closure of the defect.
Results:
Forty-three out of 45 patients had successful device closure. The mean age and weight were 25.71 ± 8.62 months and 8.99 ± 1.24 kg, respectively. The defect measuring as large as 27 mm (14.89 ± 3.89) on TEE was closed and device as big as 28 mm was successfully deployed (16.7 ± 4.31). Regular technique of device deployment was successful in only 15 cases. In the remaining 28, one of the modified techniques was used. There was no mortality, failure of the procedure, device embolization, thromboembolism, or pericardial effusion. One patient developed moderate mitral regurgitation and two patients had transient atrioventricular block. At follow-up, all patients showed significant improvement in symptoms and growth without any complications.
Conclusions:
Defect size as large as three times the weight in kg can be closed in small children. Devices as large as 28 mm can be deployed in these hearts provided the surrounding rims are adequate. In majority of cases, one of the modified techniques is essential for successful deployment. IAS length is not a limiting factor for deciding the size of the device used.
Keywords: Amplazer septal occluder, atrial septal defect, interatrial septal length, techniques of deployment
INTRODUCTION
Atrial septal defect (ASD) constitutes 8%–10% of the congenital heart defects in children. The secundum ASD accounts for nearly 75% of all ASDs.[1] Since the introduction of transcatheter device closure for secundum ASDs in 1976 by King et al.,[2] there has been a paradigm shift in their management. Over the years, the procedure has evolved significantly to become a treatment of choice in many institutions. The Amplatzer septal occluder (ASO) is the most widely used device owing to its user-friendliness and high success rate.[3] Various studies have reported transcatheter closure to be as effective, and with lower complication rate, as compared to surgical closure.[4,5] However, most of these studies have included bigger children, adolescents, and adults.[4,5,6] Although a few studies have demonstrated the feasibility and reasonable safety of transcatheter ASD device closure in very young children,[7,8,9,10] none of them have addressed important issues like how large a defect is too large for device closure, how to select the size of the device, does the length of the interatrial septum (IAS) matter in the device selection, and is there a need for using modified techniques to achieve successful deployment of the device in this subset of patients which is characterized by relatively large defects in small hearts.
In this study, we report 45 children weighing ≤10 kg who were considered suitable for percutaneous ASD device closure with a special emphasis on defect size, patient and device selection, and technical modifications for a successful device deployment along with the follow-up data pertaining to their safety and efficacy. This is perhaps the largest cohort of children weighing ≤10 kg undergoing device closure with the longest follow-up.
MATERIALS AND METHODS
Between June 2010 and January 2018, a total of 45 children weighing ≤10 kg, were selected for percutaneous ASD device closure.
After obtaining a detailed history from the parents, all the children underwent thorough physical examination, a baseline electrocardiogram (ECG), and chest X-ray. A transthoracic echo (TTE) was performed, and the diameter of the ASD and length of all the rims were measured in multiple planes from different views. The associated valvular lesions or additional shunts were noted. The pulmonary artery pressure was estimated taking into consideration the peak velocity of tricuspid regurgitation jet by continuous-wave Doppler and using modified Bernoulli equation.
The selection criteria for the device closure at such a young age and small weight were symptomatic status, presence of pulmonary hypertension, hemodynamically significant left-to-right shunt, adequacy of the rims on the TTE (superior vena cava [SVC] rim, inferior vena cava [IVC] rim and posterior rim >4 mm; AV valve or mitral rim >7 mm), and absence of any other cardiac lesion requiring surgical intervention. The aortic rim was deficient in majority of cases. As for the symptoms, failure to thrive (FTT) was defined as weight for age ≤5th percentile.[11] Frequent respiratory tract infections (RTI's) were defined as ≥6 events per year (or part thereof) requiring antibiotics.[12] Size of the defect vis-a-vis the age and weight of the child, no matter how large, was not considered as a contraindication for the device closure as long as the surrounding rims were found to be adequate. Informed written consent was obtained from the parents.
The procedure was done under general anesthesia. All the children underwent transesophageal echocardiography (TEE) for reassessment of the defect size and surrounding margins prior to device closure. The ASO was used in all the cases. The device size was selected based on the maximum diameter of the ASD as determined on TEE at 0°, 45°, and 90°. The ASO used was either equal to or about 10% more than the maximum ASD diameter. Balloon sizing was not done in any of the patients. The length of the IAS was measured at 0° and 90° and the longer of the two measurements was used to define the length. Although the IAS length was estimated, it did not determine the maximum size of the device to be used. After obtaining the venous access, heparin was administered in the dose of 100 i.u/kg. Thereafter, 50 i.u./kg of heparin was administered every 30 min if the procedure time extended beyond 60 min. Activated clotting time was not monitored during the procedure. Intravenous (IV) antibiotic was given 1 h before and 8 and 24 h after the procedure. Postprocedure, children were observed for 24 h and were discharged on oral aspirin in the dose of 5 mg/kg/day for 6 months. All of them underwent predischarge ECG and TTE.
Children were followed at 6 weeks, 6 months, and yearly thereafter to assess the symptomatic status, catchup growth, device status, residual shunt, reversed remodeling of the right heart, and for the occurrence of any complication.
RESULTS
Patient characteristics and indications for closure are summarized in Table 1. The youngest child was 8 months, while the smallest weighed only 5.7 kg. Repeated RTIs and significant FTT were the most common indications for ASD closure at such a young age. Surprisingly, none of the patients had congestive cardiac failure.
Table 1.
Patient characteristics and indications
| Characteristic/indications | Number/value |
|---|---|
| Total number of patients | 45 |
| Gender (male/female) | 20/25 |
| Age (months) | 26 (8-38) |
| Weight (kg) | 9.65 (5.7-10) |
| Indications of a | |
| FTT | 16 |
| RTIs | 9 |
| FTT with RTIs | 15 |
| Developmental/chromosomal abnormality | 5 |
| Mild (PAH) | 2 |
| Congestive heart failure (CCF) | 0 |
FTT: Failure to thrive, RTIs: Recurrent respiratory tract infections, PAH: Pulmonary arterial hypertension, CCF: Congestive cardiac failure
Associated lesions: These have been summarized in Table 2. About one out of every four of these patients had associated cardiac lesions. However, only one of them with a patent ductus arteriosus underwent device closure in the same sitting, while in the others, the lesions were found to be hemodynamically insignificant and were, therefore, left alone.
Table 2.
Associated lesions
| Lesions | Number of cases |
|---|---|
| Valvular pulmonary stenosis | 7 |
| PDA | 1 |
| Right lower pulmonary vein stenosis | 1 |
| VSD | 1 |
PDA: Patent ductus arteriosus, VSD: Ventricular septal defect
In two patients who had pulmonary arterial hypertension (PAH) on TTE, PA pressures were estimated at cardiac catheterization and were found to be less than half of the systemic pressures. They underwent device closure without any tests for reversibility of PAH.
The defect characteristics and the procedural technique used are summarized in Table 3. During the TEE evaluation, one patient was found to have a deficient and floppy IVC rim, while the other had a deficient SVC rim. Hence, they were advised to undergo surgical closure of their ASD. The procedure was successful in rest of the 43 cases. Two patients had fenestrated secundum ASD which was closed using a single device since the fenestration was placed in the immediate vicinity of the main defect. On TEE, the median defect size was found to be 15 mm (9–27 mm). The defect size (in mm) to weight (in kg) ratio was >2:1 in 11, >1.5:1 in 17, and >1.2:1 in 11 patients. The mean IAS length was 30.6 ± 3.75 mm (22–37 mm), and in nearly 40% (17/43 cases) of the total study group, the left atrial (LA) disc size was found to be greater than the IAS length.
Table 3.
Defect characteristics, procedural technique, and complications
| Characteristics/techniques/complications | Number/value |
|---|---|
| Successful device closure | 43 cases |
| Defect to weight ratio, mean | 1.65±0.42 (0.9-2.81) |
| Defect size (mm), median | 15 (9-27) |
| Device size (mm), median | 16 (10-28) |
| IAS length (mm), median | 30.60 (22-37) |
| Device to defect ratio, mean | 1.14±0.04 (1.04-1.20) |
| Device to weight ratios, mean | 1.88±0.46 (1.00-2.92) |
| Techniques | |
| Left superior pulmonary vein | 18 cases |
| Right superior pulmonary vein | 3 cases |
| Balloon assisted | 6 cases |
| Left atrial appendage | 1 case |
| Regular technique | 15 cases |
| Complications | |
| Transient Mobitz Type I block | 1 |
| 2:1 AV block | 1 |
| Grade II Mitral regurgitation | 1 |
IAS: Interatrial septum, AV: Atriovetricular
ASO (Abbott, USA) was used for closing the defect in all. The device to defect ratio in the study group was 1.14 ± 0.04 (1.04–1.2). The various techniques of device deployment, included the left upper pulmonary vein technique in 42% cases, regular in 35%, balloon assisted in 14%, right pulmonary vein in 7%, and left atrial disc engagement-disengagement technique (LADEDT) using the left atrial appendage for engagement in 2%. In all the three patients where right upper pulmonary vein deployment technique was successfully used, there was a failed attempt with the left upper pulmonary venous technique. Out of 11 patients who had defect to weight ratio ≥2.0, all needed a modified technique with three requiring balloon-assisted device closure.
There were three complications related to the procedure. Two cases had rhythm disturbance, one developed transient Mobitz Type 1 atrioventricular (AV) block immediately after the device placement which reverted to normal AV conduction with the use of IV steroids and atropine. The other child had 2:1 AV block after 24 h of device deployment which reverted to 1:1 conduction after 48 h of oral steroids and nonsteroidal anti-inflammatory drug (NSAID) treatment. The third case developed Grade II mitral regurgitation (MR) at the time of deployment due to trauma to the anterior mitral leaflet (AML). The parents refused surgical removal of the device with repair of the mitral valve. During the follow-up, she continues to have moderate MR with mild left atrial and left ventricular enlargement with normal left ventricular contractility.
The mean follow-up period was 18.5 ± 17.9 months with median of 12 months. Out of 43 patients, 41 had catch-up weight above the fifth percentile for age, on follow-up [Figure 1]. None of the children had repeated RTI following the device closure. Apart from the frequency, the severity of RTI in terms of their duration and response to antibiotics, came down remarkably. The reversed remodeling of the right atrium and the right ventricle were subjectively seen in all, but no objective estimates of the right-sided structures were made. There were no delayed complications in the form of device embolization, thromboembolism, residual shunt, cardiac erosion, or heart block.
Figure 1.
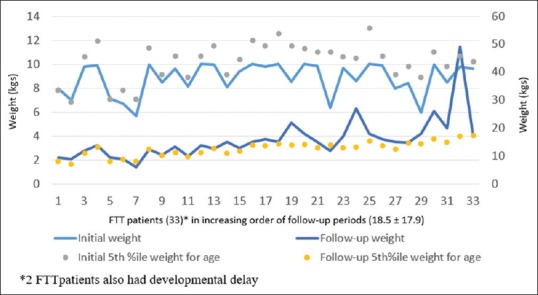
Follow-up weights of children with failure to thrive after device closure
DISCUSSION
Age and weight of closure
In asymptomatic or minimally symptomatic children, ASD device closure is routinely done in the preschool going age of around 4 years with children weighing about 15 kg.[13,14,15,16] However, in this study, the mean age (25.71 months) and weight (8.99 kg) were much less in view of the symptomatic status of these children with or without elevation of the pulmonary arterial pressures (PAP). Recent studies have adequately documented the feasibility of device placement in this subset, but the complication rate has been significant, especially in those who were small and very sick due to bronchopulmonary dysplasia.[7,8,9,10,17] In some earlier studies, such smaller children as a relative contraindication for device closure and suggested surgery.[18,19,20] In the present study, we have confirmed the feasibility of the closure but have also established the safety of the procedure.
Indications for early closure
Majority of children with ASD are either asymptomatic or minimally symptomatic in infancy and early childhood despite large shunt size.[21,22] However, in some, large left-to-right shunt can result in repeated RTIs requiring frequent use of antibiotics or even hospitalization, significant FTT, frequent episodes of wheezing requiring visits to the emergency room for nebulization, presence of heart failure, or pulmonary hypertension.[7,8,9,10,23,24,25] The entire cohort, which is a subject of the present study, required early device closure due to one or more of the above-mentioned indications. Surprisingly, none of them had heart failure despite large defect size.
Choosing device size in small children
There is no standard rule to determine the device size for ASD closure. Some groups insist on balloon sizing using stop-flow technique,[10,17,26] while the others measure the ASD size on TEE in various planes to determine the device size.[27,28] In the present study, we have used the device size which was 0%–10% more than the maximum ASD size on TEE at 0°, 45°, and 90°. Some of the previous reports have recommended estimation of the length of the IAS and have avoided using a device whose left atrial disc length was greater than this length.[19] In this study, 26/43 children had a septal length which was more than or equal to LA disc length. In the remaining, despite LA disc length being more than the length of the IAS, the ASDs were closed successfully without compromising on the procedural safety in the short and intermediate term. This confirmed our belief that the echocardiographic assessment of the length of the IAS is likely to be underestimated on TEE probably due to the fact that its lie is not in one single plane. Furthermore, the ASO is sufficiently pliable to conform itself to the plane of the IAS in a curvilinear fashion. Therefore echo measurement of the IAS length should not be taken into consideration for selecting the maximum device size to be used in children.
How large atrial septal defect is too large for device closure
Although some of the earlier publications have recommended against using devices which are 150%–200% more than the weight of the child in kgs, such recommendations have been empirical.[19,29] In the present study, device size as large as up to 300% of the body weight has been used safely and effectively. Logically speaking, children with large defects and adequate rim length (≥4 mm) are likely to have adequate heart size and septal length to accommodate correspondingly large devices, irrespective of their weight and age. In the present study, 35% had devices ≥150% and 44% had devices ≥200% of their weight in kg. This clearly shows that the size of the device vis-a-vis the weight or age of the child is not a limitation for device closure.
Why the technical modifications
Large ASD in children weighing ≤10 kg is associated with a small left atrial size resulting in its inability to accommodate the opened up left atrial disk. This lack of accommodation secondarily results in the malalignment of the LA disk with the plane of the IAS resulting in prolapse of the device across the defect. As a result, routine technique of device deployment[30] is likely to fail. Early in the course of this study, we made a number of attempts using the routine technique because LADEDT group of techniques was not standardized. Balloon-assisted technique (BAT) using Equalizer balloon (Boston Scientific, Natick, MA, USA) in this subset had problems related to introduction of the balloon due to its blunt tip and very high profile. For the same reasons, achieving hemostasis was also difficult and prolonged. These problems were overcome with the use of Amplatzer sizing balloon during the device delivery and the technique was used safely, effectively, and with much more ease in terms of balloon introduction and achieving hemostasis. Once the alternative techniques were standardized,[31,32,33] they were used to begin with while dealing with small children, rather than using them if the usual technique failed. Almost two-thirds (28/43) children in this study underwent successful device closure using one of the alternative techniques. Among these, the left superior pulmonary vein technique was most commonly used being simple to execute. However, BAT, which was used in six patients, was successful in all, wherein the attempts with the other techniques had failed. The successful deployment was possible in 100% of the patients with the use of either the routine or one of the modified techniques. Our data show that the device closure in small children is not only feasible, but it can be done with a great degree of predictability.
Efficacy
As seen in the earlier reports, all the children improved symptomatically with the catch-up growth being seen in 95% of the patients.[8,9,10] Reversed remodeling of the right heart was obvious even at the time of the first follow-up visit and by 6 months, all the patients had normalization of the right atrium the right ventricle as reported by others.,[34,35] This reversed remodeling of the right heart was judged subjectively without any objective assessment of right atrial or right ventricular size or volume. Bartakian et al. have found no difference in the rate of resolution of right heart enlargement after device closure in children weighing <15 kg.[17]
Safety
Only 1/43 patients who underwent successful device closure developed moderate MR which did not progress till the time of the last follow-up. This child required multiple attempts in order to achieve appropriate device deployment. During one of these attempts, she developed MR probably due to LA disc traumatizing the AML. Although some of the large devices extended up to the base of the AML sometimes even making a contact with the leaflet, there was no new occurrence of MR either acutely or over a period of time. In spite of the reversed remodeling, the growth of these children made the devices move away from the AML, reducing the chance of future occurrence of MR. However, AV valve regurgitation remains a potential complication with the use of large devices in children as reported before.[17,36,37]
Two additional patients developed AV block. One had transient Mobitz type-I AV block after device placement which reverted spontaneously in the catheterization laboratory with the use of IV corticosteroids and atropine. The other child developed a 2:1 AV block after 24 h which reverted to 1:1 conduction after 48 h of corticosteroid and NSAID treatment. Although most of the reports of heart block are in the adult population,[38,39,40] children are not immune to it as was demonstrated by these 2 cases. Some of them revert to 1:1 AV conduction with medical therapy as it occurred in our patients, while the others need surgical removal of the device.[41,42] If the device is removed early, majority regain normal AV conduction.[41,42]
Some studies have shown higher rate of major complications; however, this could be because of low sample size used in these studies and a sicker cohort.[7,8] In our study, no major complications were seen either acutely or in the intermediate-term follow-up. Although device embolization is reported more often while closing large defects, it is the deficient margin (s) associated with these large defects or undersizing of the device which are the real culprits. Appropriate patient selection in terms of adequate margin length and device selection in terms of its relation to the largest diameter, helped in avoiding device embolization as has been shown in the earlier reports.[8,13,19] Very rarely, insufficient rim strength can result in device embolization despite adequately long rims.[43,44] Fortunately, none of our children had a floppy rim incapable of supporting the device.
The median follow-up period for the study is about 12 months. The authors will publish 5-year follow-up data in due course for examining any complications on long-term basis.
CONCLUSIONS
Feasibility, efficacy, and safety of device closure of ASDs in small children weighing <10 kg is reaffirmed in this study. Defect size up to 300% of the weight in kg can be closed with a device of appropriate size so long as the defect rims are adequate. TEE measurement of the defect diameter in three standard views is adequate to decide the device size. The length of the IAS is not a limiting factor for deciding the size of the device since devices with LA disc longer than the length of the IAS can be accommodated in these small hearts. In view of the problems of accommodation of the opened up LA disc within these small left atria, one of the modified techniques is necessary in order to achieve the successful deployment of the device. Although there are finite complications, their incidence and severity are low. Based on our data, we recommend that children requiring ASD closure much before the traditional age and weight due to their clinical and hemodynamic status should be offered device therapy.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Allen HD, Driscoll DJ, Shaddy RE, Feltes TF. Moss and Adams Heart Disease in Infants, Children and Adolescents Including the Fetus and Young Adult. 8th ed. Philadelphia: Lippincott Williams & Wilkins; 2013. p. 672. [Google Scholar]
- 2.King TD, Thompson SL, Steiner C, Mills NL. Secundum atrial septal defect Nonoperative closure during cardiac catheterization. JAMA. 1976;235:2506–9. [PubMed] [Google Scholar]
- 3.Horst S, Qureshi SA, Wilson N, Hizaji ZM. Percutaneous Interventions for Congenital Heart Disease. UK: Informa Heathcare; 2007. pp. 266–72. [Google Scholar]
- 4.Du ZD, Hijazi ZM, Kleinman CS, Silverman NH, Larntz K Amplatzer Investigators. Comparison between transcatheter and surgical closure of secundum atrial septal defect in children and adults: Results of a multicenter nonrandomized trial. J Am Coll Cardiol. 2002;39:1836–44. doi: 10.1016/s0735-1097(02)01862-4. [DOI] [PubMed] [Google Scholar]
- 5.Berger F, Vogel M, Alexi-Meskishvili V, Lange PE. Comparison of results and complications of surgical and Amplatzer device closure of atrial septal defects. J Thorac Cardiovasc Surg. 1999;118:674–8. doi: 10.1016/S0022-5223(99)70013-9. [DOI] [PubMed] [Google Scholar]
- 6.Carminati M, Chessa M, Butera G, Bini RM, Giusti S, Festa P, et al. Transcatheter closure of atrial septal defects with the STARFlex device: Early results and follow-up. J Interv Cardiol. 2001;14:319–24. doi: 10.1111/j.1540-8183.2001.tb00339.x. [DOI] [PubMed] [Google Scholar]
- 7.Vogel M, Berger F, Dähnert I, Ewert P, Lange PE. Treatment of atrial septal defects in symptomatic children aged less than 2 years of age using the Amplatzer septal occluder. Cardiol Young. 2000;10:534–7. doi: 10.1017/s1047951100008234. [DOI] [PubMed] [Google Scholar]
- 8.Diab KA, Cao QL, Bacha EA, Hijazi ZM. Device closure of atrial septal defects with the Amplatzer septal occluder: Safety and outcome in infants. J Thorac Cardiovasc Surg. 2007;134:960–6. doi: 10.1016/j.jtcvs.2007.06.018. [DOI] [PubMed] [Google Scholar]
- 9.Wyss Y, Quandt D, Weber R, Stiasny B, Weber B, Knirsch W, et al. Interventional closure of secundum type atrial septal defects in infants less than 10 kilograms: Indications and procedural outcome. J Interv Cardiol. 2016;29:646–53. doi: 10.1111/joic.12328. [DOI] [PubMed] [Google Scholar]
- 10.Knop M, Szkutnik M, Fiszer R, Białkowska B, Głowacki J, Białkowski J. Transcatheter closure of atrial septal defect in children up to 10 kg of body weight with Amplatzer device. Cardiol J. 2014;21:279–83. doi: 10.5603/CJ.a2013.0120. [DOI] [PubMed] [Google Scholar]
- 11.Hamill PV, Drizd TA, Johnson CL, Reed RB, Roche AF, Moore WM. Physical growth: National center for health statistics percentiles. Am J Clin Nutr. 1979;32:607–29. doi: 10.1093/ajcn/32.3.607. [DOI] [PubMed] [Google Scholar]
- 12.Burgio GR. Il bambino con infezioniricorrenti. In: Plebani A, editor. Immunologia Pediatrica. Milan: McGraw Hill Libri Italia; 1998. p. 17. [Google Scholar]
- 13.Feltes TF, Bacha E, Beekman RH, 3rd, Cheatham JP, Feinstein JA, Gomes AS, et al. Indications for cardiac catheterization and intervention in pediatric cardiac disease: A scientific statement from the American Heart Association. Circulation. 2011;123:2607–52. doi: 10.1161/CIR.0b013e31821b1f10. [DOI] [PubMed] [Google Scholar]
- 14.Castaneda AR, Jonas RA, Mayer JE, Hanley FL, editors. Cardiac Surgery of the Neonate and Infants. Philadelphia, PA: WB Saunders; 1994. Atrial septal defect; p. 143. [Google Scholar]
- 15.Kirklin JW, Barrat-Boyes BG. Cardiac Surgery. 2nd ed. New York: Churchill Livingstone; 1993. p. 609. [Google Scholar]
- 16.Keane JF, Geva T, Fyler DC. Atrial septal defect. In: Keane JF, Lock JE, Fyler DC, editors. Nadas Pediatric Cardiology. Philadelphia: Saunders Elsevier; 2006. pp. 603–16. [Google Scholar]
- 17.Bartakian S, Fagan TE, Schaffer MS, Darst JR. Device closure of secundum atrial septal defects in children <15kg: Complication rates and indications for referral. JACC Cardiovasc Interv. 2012;5:1178–84. doi: 10.1016/j.jcin.2012.07.009. [DOI] [PubMed] [Google Scholar]
- 18.Petit CJ, Justino H, Pignatelli RH, Crystal MA, Payne WA, Ing FF. Percutaneous atrial septal defect closure in infants and toddlers: Predictors of success. Pediatr Cardiol. 2013;34:220–5. doi: 10.1007/s00246-012-0413-6. [DOI] [PubMed] [Google Scholar]
- 19.Butera G, De Rosa G, Chessa M, Rosti L, Negura DG, Luciane P, et al. Transcatheter closure of atrial septal defect in young children: Results and follow-up. J Am Coll Cardiol. 2003;42:241–5. doi: 10.1016/s0735-1097(03)00589-8. [DOI] [PubMed] [Google Scholar]
- 20.Rastogi N, Smeeton NC, Qureshi SA. Factors related to successful transcatheter closure of atrial septal defects using the Amplatzer septal occluder. Pediatr Cardiol. 2009;30:888–92. doi: 10.1007/s00246-009-9452-z. [DOI] [PubMed] [Google Scholar]
- 21.Kavanagh-Gray D. Atrial septal defect in infancy. Can Med Assoc J. 1963;89:491–9. [PMC free article] [PubMed] [Google Scholar]
- 22.Nakamura FF, Hauck AJ, Nadas AS. Atrial septal defect in infants. Pediatrics. 1964;34:101–6. [PubMed] [Google Scholar]
- 23.Phillips SJ, Okies JE, Henken D, Sunderland CO, Starr A. Complex of secundum atrial septal defect and congestive heart failure in infants. J Thorac Cardiovasc Surg. 1975;70:696–700. [PubMed] [Google Scholar]
- 24.Hunt CE, Lucas RV., Jr Symptomatic atrial septal defect in infancy. Circulation. 1973;47:1042–8. doi: 10.1161/01.cir.47.5.1042. [DOI] [PubMed] [Google Scholar]
- 25.Dimich I, Steinfeld L, Park SC. Symptomatic atrial septal defect in infants. Am Heart J. 1973;85:601–4. doi: 10.1016/0002-8703(73)90164-6. [DOI] [PubMed] [Google Scholar]
- 26.Hijazi ZM. Device closure of secundum atrial septal defects: To balloon size or not to balloon size. Ann Pediatr Cardiol. 2011;4:34–5. [PMC free article] [PubMed] [Google Scholar]
- 27.Mazic U, Gavora P, Masura J. The role of transesophageal echocardiography in transcatheter closure of secundum atrial septal defects by the Amplatzer septal occluder. Am Heart J. 2001;142:482–8. doi: 10.1067/mhj.2001.116770. [DOI] [PubMed] [Google Scholar]
- 28.Vaidyanathan B, Simpson JM, Kumar RK. Transesophageal echocardiography for device closure of atrial septal defects: Case selection, planning, and procedural guidance. JACC Cardiovasc Imaging. 2009;2:1238–42. doi: 10.1016/j.jcmg.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 29.Justino H. Transcatheter device closure of atrial septal defects in small children: Sound judgment is key. Rev Bras Cardiol Invasiva. 2013;21:101–2. [Google Scholar]
- 30.Pillai AA, Satheesh S, Pakkirisamy G, Selvaraj R, Jayaraman B. Techniques and outcomes of transcatheter closure of complex atrial septal defects – Single center experience. Indian Heart J. 2014;66:38–44. doi: 10.1016/j.ihj.2013.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pinto R, Jain S, Dalvi B. Transcatheter closure of large atrial septal defects in children using the left atrial disc engagement-disengagement technique (LADEDT)-technical considerations and short term results. Catheter Cardiovasc Interv. 2013;82:935–43. doi: 10.1002/ccd.24873. [DOI] [PubMed] [Google Scholar]
- 32.Dalvi B. Balloon assisted technique for closure of large atrial septal defects. Images Paediatr Cardiol. 2008;10:5–9. [PMC free article] [PubMed] [Google Scholar]
- 33.Dalvi B, Pinto R, Gupta A. Device closure of large atrial septal defects requiring devices>or=20 mm in small children weighing<20 kg. Catheter Cardiovasc Interv. 2008;71:679–86. doi: 10.1002/ccd.21450. [DOI] [PubMed] [Google Scholar]
- 34.Ammar A, Arab TM, Hammady WA, Sayed MH. Intermediate outcome of transcatheter closure of secundum atrial septal defect on cardiac remodeling in Egyptian children and adults. J Cardiol Curr Res. 2016;6:00224. [Google Scholar]
- 35.Kaya MG, Baykan A, Dogan A, Inanc T, Gunebakmaz O, Dogdu O, et al. Intermediate-term effects of transcatheter secundum atrial septal defect closure on cardiac remodeling in children and adults. Pediatr Cardiol. 2010;31:474–82. doi: 10.1007/s00246-009-9623-y. [DOI] [PubMed] [Google Scholar]
- 36.Behjati M, Mirhosseini SJ, Hosseini SH, Rajaei S. Transcatheter closure of atrial septal defect with Amplatzer device in children and adolescents: Short and midterm results; an Iranian experience. Iran J Pediatr. 2011;21:166–72. [PMC free article] [PubMed] [Google Scholar]
- 37.Sadiq N, Ullah M, Sultan M, Akhtar K, Akbar H. Transcatheter device closure of secundum atrial septal defect (ASD) in young children. Pak Armed Forces Med J. 2014;64:355–9. [Google Scholar]
- 38.Chessa M, Carminati M, Butera G, Bini RM, Drago M, Rosti L, et al. Early and late complications associated with transcatheter occlusion of secundum atrial septal defect. J Am Coll Cardiol. 2002;39:1061–5. doi: 10.1016/s0735-1097(02)01711-4. [DOI] [PubMed] [Google Scholar]
- 39.Wang Y, Hua Y, Li L, Wang X, Qiao L, Shi X, et al. Risk factors and prognosis of atrioventricular block after atrial septum defect closure using the Amplatzer device. Pediatr Cardiol. 2014;35:550–5. doi: 10.1007/s00246-013-0822-1. [DOI] [PubMed] [Google Scholar]
- 40.Al-Anani SJ, Weber H, Hijazi ZM. Atrioventricular block after transcatheter ASD closure using the Amplatzer septal occluder: Risk factors and recommendations. Catheter Cardiovasc Interv. 2010;75:767–72. doi: 10.1002/ccd.22359. [DOI] [PubMed] [Google Scholar]
- 41.Clark JB, Chowdhury D, Pauliks LB, Weber HS. Resolution of heart block after surgical removal of an Amplatzer device. Ann Thorac Surg. 2010;89:1631–3. doi: 10.1016/j.athoracsur.2009.09.089. [DOI] [PubMed] [Google Scholar]
- 42.Suda K, Raboisson MJ, Piette E, Dahdah NS, Miró J. Reversible atrioventricular block associated with closure of atrial septal defects using the Amplatzer device. J Am Coll Cardiol. 2004;43:1677–82. doi: 10.1016/j.jacc.2003.12.042. [DOI] [PubMed] [Google Scholar]
- 43.Son JW, Park JS. Subacute, silent embolization of Amplatzer atrial septal defect closure device to the pulmonary artery. J Cardiovasc Ultrasound. 2012;20:201–4. doi: 10.4250/jcu.2012.20.4.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hizaji ZM, Ted F, John PC, Horst S. Complications during Percutaneous Interventions for Structural and Congenital Heart Disease. UK: Informa Healthcare; 2009. p. 211. [Google Scholar]


