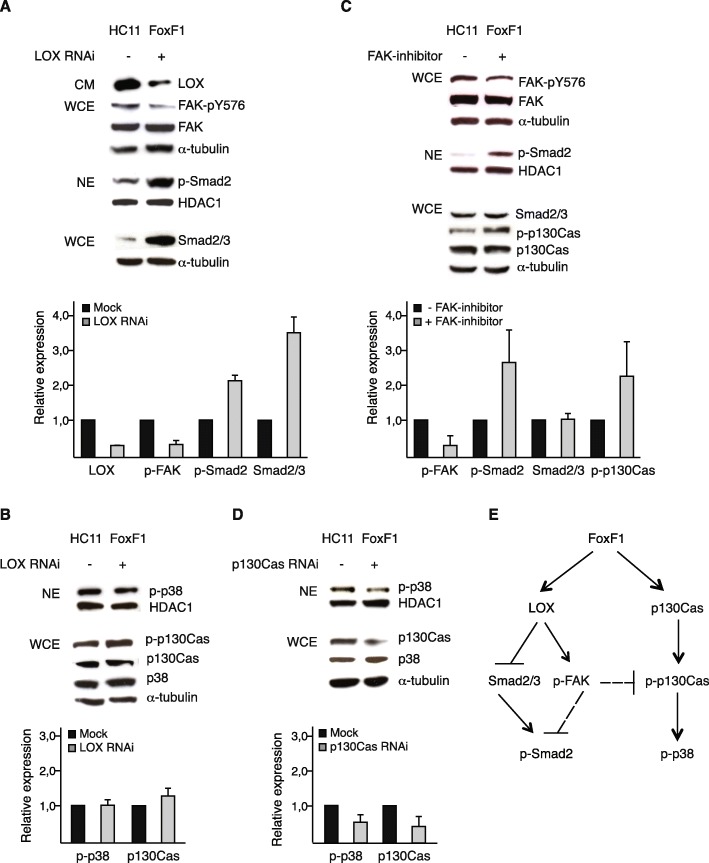
Fig. 6.
FoxF1 represses Smad2 by a LOX- and FAK-dependent mechanism. a-b, upper panels, western blot analysis of HC11FoxF1 cells, mock-treated or transfected with LOX siRNA. Supernatants of cultures (CM) were probed with LOX antibody, whole cell extracts (WCE) were probed with phosphospecific antibody FAK-pY576 and α-tubulin antibodies, stripped and re-probed with FAK antibody. Nuclear extracts (NE) were probed with phosphospecific Smad2 antibody, stripped and re-probed with HDAC-1 antibody. Total levels of Smad2/3 were analyzed in whole cell extracts (a). Nuclear extracts were probed with phosphospecific p38 and HDAC-1 antibodies. Whole cell extracts were probed with phosphospecific p130Cas and p38 antibodies, stripped and re-probed with p130Cas and α-tubulin antibodies (b). c, upper panel, western blot analysis of HC11FoxF1 cells untreated or treated with FAK inhibitor (FAK inhibitor 14, Santa Cruz) 20 μM for 1 h. The effect of the FAK inhibitor was confirmed by analyzing FAK phosphorylation levels at Y396 (data not shown). Whole cell extracts were probed with FAK-pY576 antibody, stripped and re-probed FAK and α-tubulin antibodies. Nuclear extracts were probed with phosphospecific Smad2 and HDAC-1 antibodies. Whole cell extracts were probed with phosphospecific p130Cas and Smad2/3 antibodies, stripped and re-probed with p130Cas and α-tubulin antibodies. d, upper panel, western blot analysis of HC11FoxF1 cells mock-treated or transfected with p130Cas siRNA. Nuclear extracts were probed with phosphospecific p38 antibody, washed, blocked and re-probed with HDAC-1 antibody. Whole cell extracts were probed with p130Cas and p38 antibodies, washed, blocked and re-probed with α-tubulin antibody. a-d, lower panels show densitometry. e, summary of signaling events regulated by FoxF1