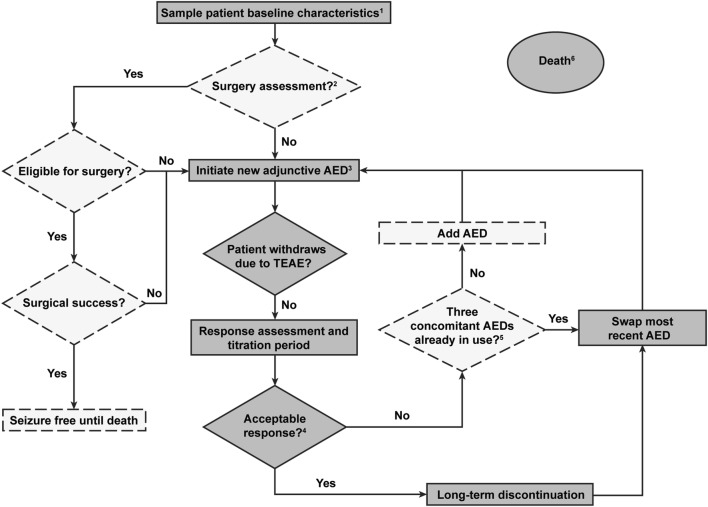
Fig. 1.
Simplified description of the discrete-event simulation model (DESM). AED antiepileptic drug, TEAE early or late onset treatment-emergent adverse event. Dashed lines denote the decisions and states excluded from the base case analysis. 1. Patient characteristics included, e.g., age, sex, seizure frequency, and ethnicity. 2. Included only in a sensitivity analysis scenario; in the base case modeling patients were assumed to have been assessed for surgery earlier based on the Finnish practice. Patients will only be assessed for the surgery once. 3. The base case modeling was initiated here, when brivaracetam or perampanel was added as a third AED on top of two base AEDs. 4. Acceptable response was seizure free or having at least a 50% reduction in seizure frequency. 5. Only relevant for a sensitivity analysis scenario. In the base case scenario, patients always had at least three concomitant AEDs. 6. Transition to death could happen at any time (absorbing, i.e., patients exit the model)