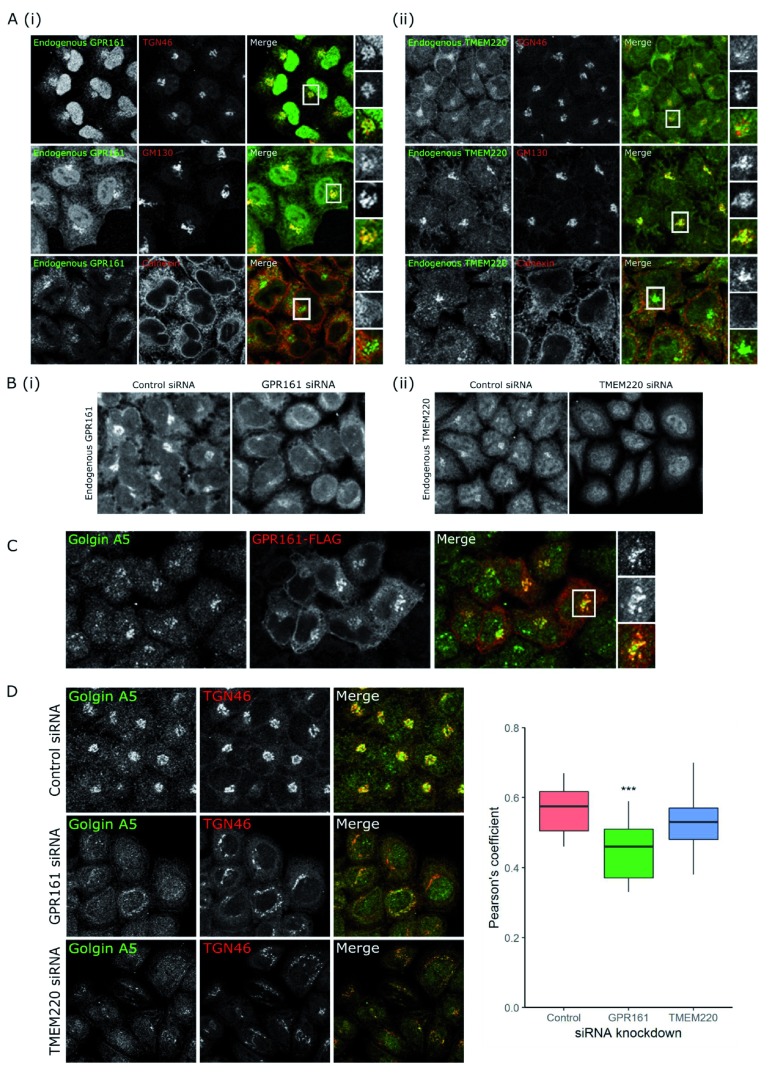
Figure 5. GPR161 and TMEM220, two novel Golgi-resident proteins involved in protein secretion, regulate glycoprotein secretion by different mechanisms.
( A) Colocalisation microscopy shows that both (i) GPR161 and (ii) TMEM220 colocalise with TGN46 and GM130, two Golgi markers, and not with calnexin, a marker of the ER. ( B) siRNA knockdown of (i) GPR161 or (ii) TMEM220 demonstrates that the antibodies used to detect these endogenous proteins only specifically detect protein at the Golgi. ( C) GPR161-FLAG (red) colocalises with golgin A5 by microscopy. ( D) Microscopy shows that knockdown of both GPR161 and TMEM220 lead to Golgi fragmentation seen by the TGN46 staining, but only GPR161 knockdown results in less colocalisation of golgin A5 and TGN46. Quantification of this change in colocalisation, measured by Pearson’s coefficient, shows that GPR161 knockdown cells have significantly less colocalisation than control cells (***p < 0.001), but there is no significant difference in colocalisation between control cells and TMEM220 knockdown cells (p = 0.302). Original magnification 20x.