Scheme 1.
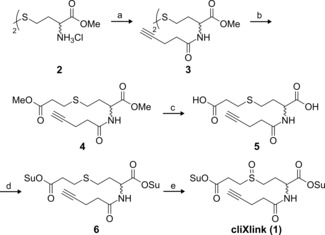
Synthesis of cliXlink (1). a) 4‐Pentynoic acid, N‐(3‐dimethylaminopropyl)‐N′‐ethylcarbodiimide hydrochloride (EDC⋅HCl), 1‐hydroxybenzotriazole hydrate (HOBt⋅H2O), NEt3, DMF, RT, 15 h, 71 %; b) over two steps: 1) tris(2‐carboxyethyl)phosphine hydrochloride (TCEP⋅HCl), NaHCO3, DMF, H2O, RT, 3 h; 2) methyl 3‐bromopropionate, 45 °C, 44 h, 55 %; c) LiOH, THF, H2O, 0 °C RT, overnight (o/n), 91 %; d) N‐hydroxysuccinimide (NHS), pyridine, trifluoroacetic anhydride, MeCN, 0 °C RT, o/n, 78 %; e) meta‐chloroperoxybenzoic acid (mCPBA), AcOEt, RT, 30 min, 57 %.
