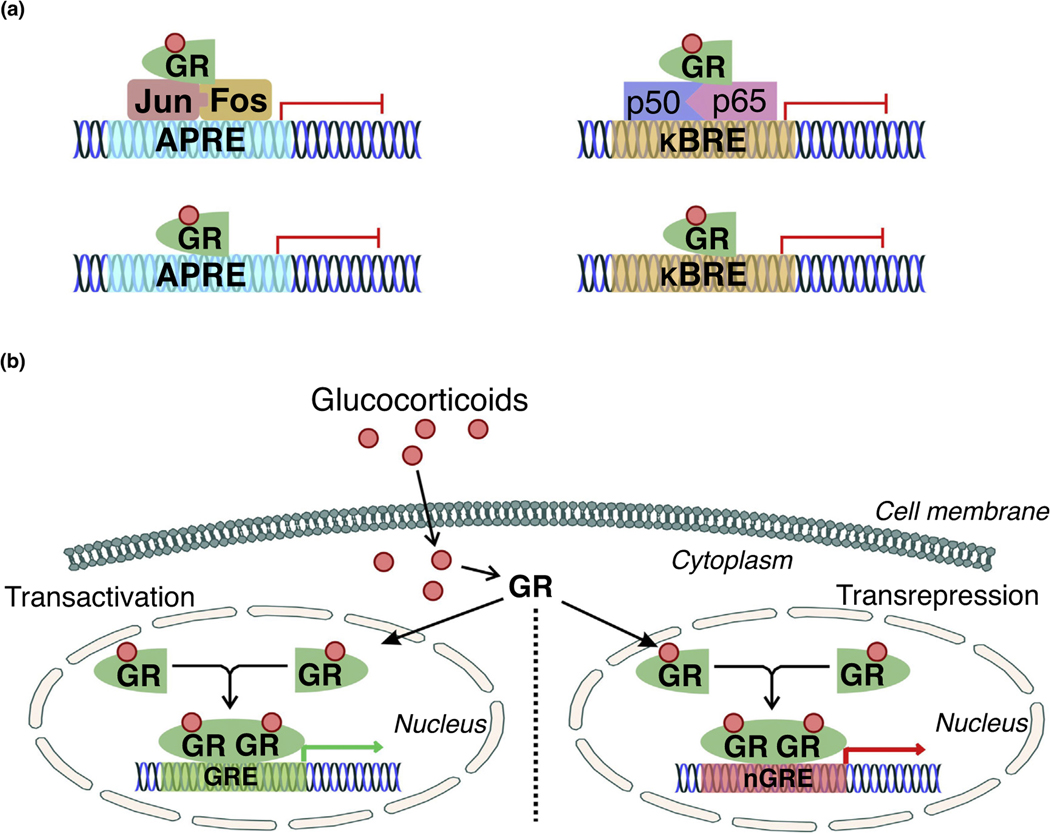
FIGURE 1.
Classical activity of glucocorticoid receptor (GR) in transactivation and transrepression. (a) Classical GR transrepression activity involves association with the activator protein-1 (AP-1, composed of the immediate early response genes Jun and Fos, which respond to cellular stimulation before protein synthesis) or nuclear factor (NF)-κB (composed of the proinflammatory p50 and p65 subunits) complexes. The GR binds to AP-1 or NF-κB to inhibit transcription by limiting their association with their respective binding regions in gene promoters (AP-1 response element, APRE; κB response element, κBRE). The GR can further bind directly to GR-binding elements in the APRE or κBRE to physically prevent the interactions of AP-1 and NF-κB with their respective response elements. (b) GR transactivation and transrepression is primarily mediated by glucocorticoids. Endogenous or exogenous glucocorticoids bind to the ligand-binding site of GR, initiating translocation to the nucleus. Dimerization between activated, ligand-bound GR monomers occurs in the nucleus, subsequently followed by binding of the dimer to glucocorticoid response elements (GREs) or negative glucocorticoid response elements (nGREs) to promote the transcription of GRE-associated genes.