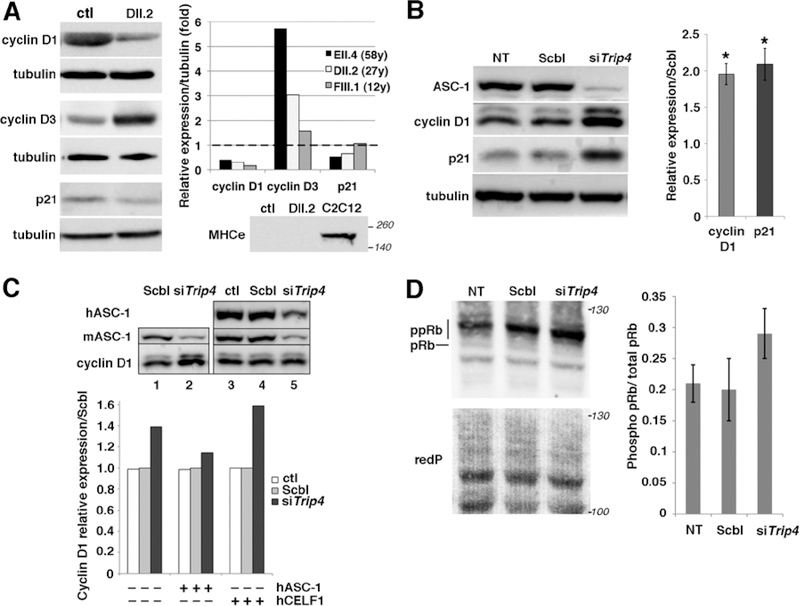
Fig. 6: Altered expression of cell cycle proteins in patient muscles and in Trip4KD myogenic cells.
(A) Western blot of lysates from control (ctl) and three patient frozen muscles revealed altered expression of cyclins D1 and D3 as well as p21 in patients DII.2 (left panel), EII.4 and FIII.1 (right panel, control expression normalized to 1, dashed line). Absence of embryonic myosin (MHCe) in both patient DII.2 and control muscle samples excluded that this might be due to active muscle regeneration (including the presence of immature myofibers) in patients (lower panel, C2C12 used as a positive control). (B) Increased cyclin D1 and p21 expression in Trip4KD C2C12 with <20% ASC-1 residual expression (data not shown) compared to non-transfected cells (NT) or cells transfected with siRNA control (Scbl) (left and right panel, normalized to Scbl transfected cells; Mann–Whitney U, *p-value <0.05). (C) Rescue of the altered levels of cyclin D1 in Trip4KD C2C12 by wild-type human ASC-1. Western blot of protein extracts from Scbl (lanes 1 and 4), siTrip4 (lanes 2 and 5) or ctl C2C12 (lane 3) double-transfected with hASC-1 (lanes 3–5) 24h after endogenous ASC-1 silencing (upper panel). Quantification of the relative expression of cyclin D1 over tubulin (normalized for Scbl) in ctl, Scbl and siTrip4 cells in the presence or absence of hASC-1. A vector expressing hCelf1 was used as a non-specific control (lower panel). mASC-1= murine endogenous ASC-1; hASC-1 = human ectopic ASC-1; hCelf1 = human ectopic Celf1. (D) Western blot analysis of pRb phosphorylation state in NT, Scbl and siTrip4 cells revealed a fast migrating band corresponding to the hypo-phosphorylated form of Rb (pRb, 110 kDa) and slower migrating bands corresponding to hyper-phosphorylated forms of Rb (ppRb, 116kDa) (left panel). Red ponceau (redP) staining of the membrane showed similar loading in each condition. In all conditions, the hyper-phosphorylated forms of Rb were predominant compared to the hypo-phosphorylated form as expected for cycling cells. Although variability precluded reaching statistical significance, quantification of the ratio ppRb/total pRb for each condition (N=4) showed an increase by nearly 50% of hyper-phosphorylated forms in siTrip4 cells (right panel).