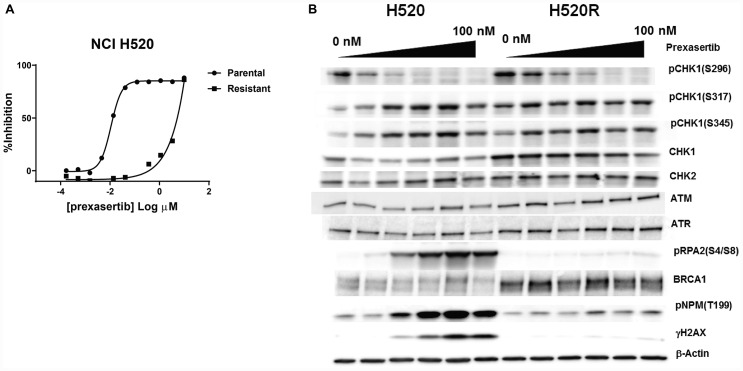
Figure 6. Pharmacological and molecular characterization of NCI-H520R cells.
(A) Viability of parental NCI-H520 and its prexasertib-resistant version (NCI-H520R) was evaluated following prexasertib treatment for 96 hours. (B) Analysis of selected cell cycle-related proteins extracted from DMSO- or prexasertib-treated cultures for 24 hours. Drug concentrations used: 0, 6.25, 12.50, 25, 50, and 100 nM.