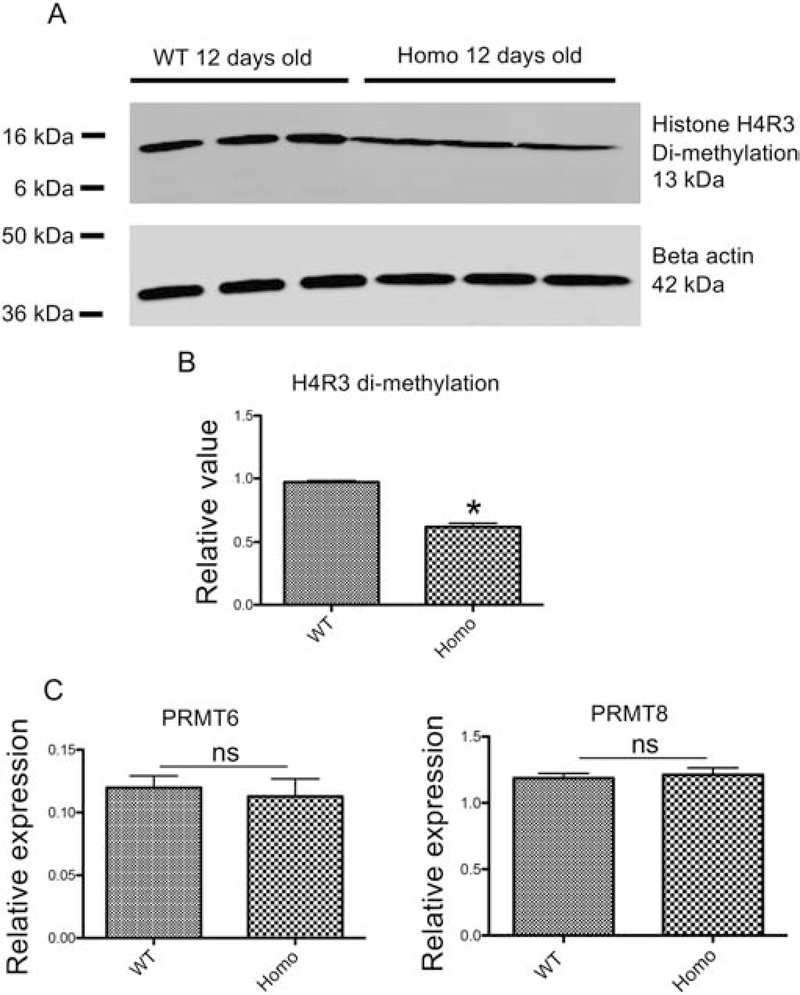
Figure 5. Histone H4R3me2a level is reduced in PRMT1 homozygous knockout tadpoles.
(A) Representative photos of western blot analysis. Proteins were isolated from three different pooled wild-type (lanes 1–3) and PRMT1 homozygous knockout (lanes 4–6) tadpoles at 12 days of age and subjected to Western blot analyses with antibodies against methylated histone H4R3 (H4R3me2a) (Upper) and beta-actin (lower) as the loading control, respectively.
(B) Quantification of histone H4R3me2a levels in wild-type and PRMT1 homozygous tadpoles. The expression levels as shown in (A) were normalized against that of beta-actin. The groups included 3 wild-type tadpoles and 4 PRMT1 homozygous tadpoles at 12 days of age, respectively. Asterisks (*) indicate a significant difference between knockout and wild-type animals (P<0.001).
(C) The expression of PRMT6 and PRMT8 is not altered in PRMT1 homozygous knockout tadpoles. Total RNA was isolated from the intestine of 10-day old wild type and PRMT1 homozygous tadpoles and used for real-time RT-PCR analysis of the expression of two PRMTs known to have H4R3me2a methylation activity: PRMT6 and PRMT8. The mRNA levels were normalized against that of rpl8. The groups included 4 wild and 5 PRMT1 homozygous knockout animals. These results were plotted with the mean, marked as a line, and SE. ns: No significant difference between knockout and wild type animals as determined by student-t test.