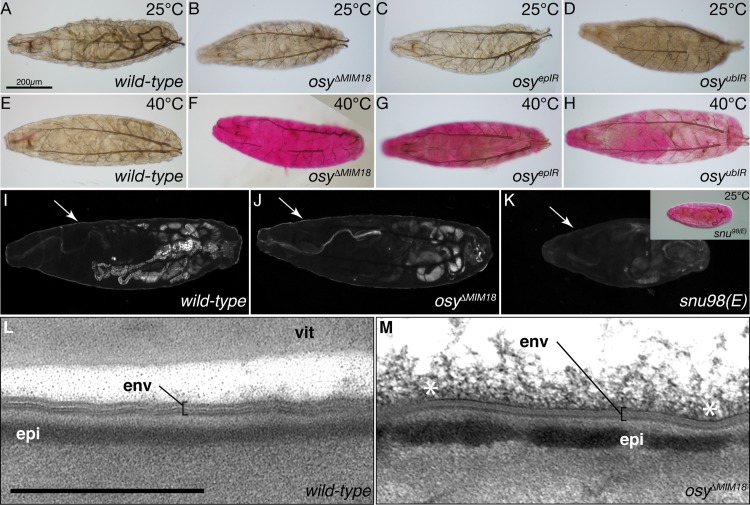
Fig 3. Osy function prevents penetration of xenobiotics.
In dye penetration assays, the 1st instar wild-type (A) and osy mutant (osyΔMiM18, B) larvae do not take up Eosin Y at 25°C Down-regulation of osy transcripts by epidermal RNAi (osyepIR, 69B-Gal4 x UAS-KK109988, C) or ubiquitous (osyubIR, da/7063-Gal4 x UAS-KK109988, D) RNAi does not cause dye penetration at 25°C in 1st instar larvae. At 40°C, the cuticle of wild-type 1st instar larvae is impermeable to Eosin Y (D), while in osy mutant 1st instar larvae the dye penetrates the cuticle (E). At 25°C, in 1st instar larvae with reduced osy transcript levels in the whole body (F) or in the epidermis (G), Eosin Y does not penetrate the cuticle. The cuticle of 1st instar larvae with reduced osy transcript levels in the whole body (H) or in the epidermis (I) fails to withstand dye penetration at 40°C. The envelope (arrow) of the wild-type 1st instar larva auto-fluoresces upon excitation with UV light (I). Envelope auto-fluorescence is unchanged in osyΔMiM18 1st instar larvae (J). By contrast, envelope auto-fluorescence is reduced in snu mutant 1st instar larvae (K). This defect allows Eosin Y penetration into these larvae at 25°C (inset). The envelope (env) of wild-type stage 17 ready-to-hatch embryos consists of alternating electron-lucid and electron-dense sheets in electron-micrographs (L). The envelope of stage 17 ready-to-hatch osyΔMiM18 embryos is normal in electron-micrographs (M). The asterisks (*) mark unspecific material at the surface of the cuticle. vit vitelline membrane. Scale bar 500nm.