Scheme 1.
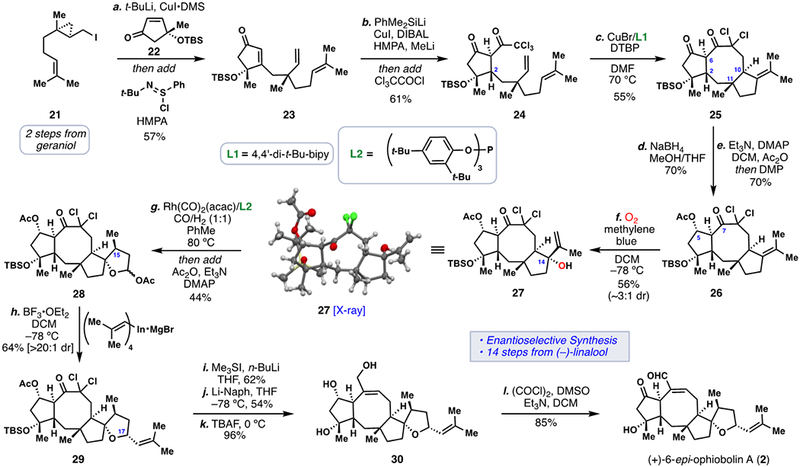
Enantioselective total synthesis of 6-epi-ophiobolin A (2). (a) 21 (1.3 equiv), t-BuLi (1.9 equiv), pentane/Et2O, –78 °C; then add CuI (0.6 equiv), DMS (2.1 equiv), 35 min; then add 22 (1.0 equiv), –78 °C to –40 °C, 4 h; then add HMPA (10.0 equiv), 10 min, –78 °C; then add N-tert-Butylbenzenesulfinimidoyl chloride (1.5 equiv), –78 °C to 25 °C, 2 h (57%); b) PhMe2SiLi, (1.25 equiv), CuI (1.0 equiv), THF, –10 °C, 10 min; then add HMPA (36.0 equiv), DIBAL (3.0 equiv), –50 °C, 30 min; then add 23 (1.0 equiv), –78 °C to –30 °C, 3 h; then add MeLi (3.8 equiv), –78 °C, 30 min; then add trichloroacetyl chloride (6.0 equiv), –78 °C to 25 °C, 3 h (61%); c) CuBr (0.7 equiv), 4,4’-di-tert-butyl-bipyridine (0.9 equiv), 2,6-di-tert-butyl-pyridine (1.0 equiv), DMF, 70 °C, 1 h (55%); d) NaBH4 (15.0 equiv), MeOH/THF (v:v = 4:1), –40 °C, 1 h (70%); e) DMAP (0.5 equiv), Et3N (2.0 equiv), Ac2O (1.0 equiv), DCM, 0 °C, 1 h; then add DMP (10.0 equiv), 0 °C to 25 °C, 1 h (70%); f) Methylene blue (0.01 equiv), O2, hν, DCM, –78 °C, 20 min; then add PPh3 (3.0 equiv), –78 °C to 25 °C, 2 h (56%, 3:1 dr); g) Rh(CO)2(acac) (0.3 equiv), tris(2,4-di-tert-butylphenyl)phosphite (0.6 equiv), 1:1 CO/H2 (100 psi), PhMe, 80 °C, 24 h; then add DMAP (0.6 equiv), Et3N (10.0 equiv), Ac2O (5.0 equiv), 0 °C to 25 °C, 1 h (44%); h) BF3•OEt2 (3.0 equiv) DCM, –78 °C, 5 min; then add In(C4H7)4•MgBr (3.0 equiv), –78 °C, 8 h (64%); i) Me3SI (20 equiv), n-BuLi (7.0 equiv), THF, –20 °C, 10 min (62%); j) Li-naphthalenide (35 equiv), THF, –78 °C, 15 min (54%); k) TBAF (5.0 equiv), THF, 0 °C, 29 h (96%); l) DMSO (15.0 equiv), (COCl)2 (10.0 equiv), DCM, –78 °C, 30 min; then add Et3N (20 equiv), –78 °C to 0 °C, 3 h (85%); DMS = dimethyl sulfide, HMPA = hexamethylphosphoramide, DMAP = 4-dimethylaminopyridine, DMP = Dess-Martin Periodinane, acac = acetylacetone, DIBAL = Diisobutylaluminium hydride, TBAF = Tetrabutylammonium fluoride, DTBP = 2,6-Di-tert-butylpyridine.
