Table 1.
Synthesis of tetracycle 29: Selected optimization.
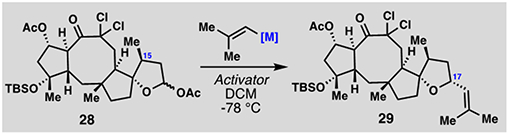 | |||
|---|---|---|---|
| Entry[a] | Activator | Nucleophile | Yield (29: 17-epi-29)[b] |
| 1 | BF3•OEt2 | MgBr(C4H7) | 36% (3:1) |
| 2 | BF3•OEt2 | ZnBr(C4H7) | 11% (2:1) |
| 3 | BF3•OEt2 | CeCl2(C4H7) | 10% (1:1.5) |
| 4 | TMSBr | CuTC(CN)(C4H7) | 17% (1:1) |
| 5 | BF3•OEt2 | InCl2(C4H7) | <5% |
| 6 | BF3•OEt2 | In(C4H7)3 | 54% (1:1) |
| 7 | BF3•OEt2 | In(C4H7)4•MgBr | 64% (>20:1) |
yields and selectivities determined by 1H NMR analysis.
yields based on amount of correct C15 diastereomer in starting 28.
