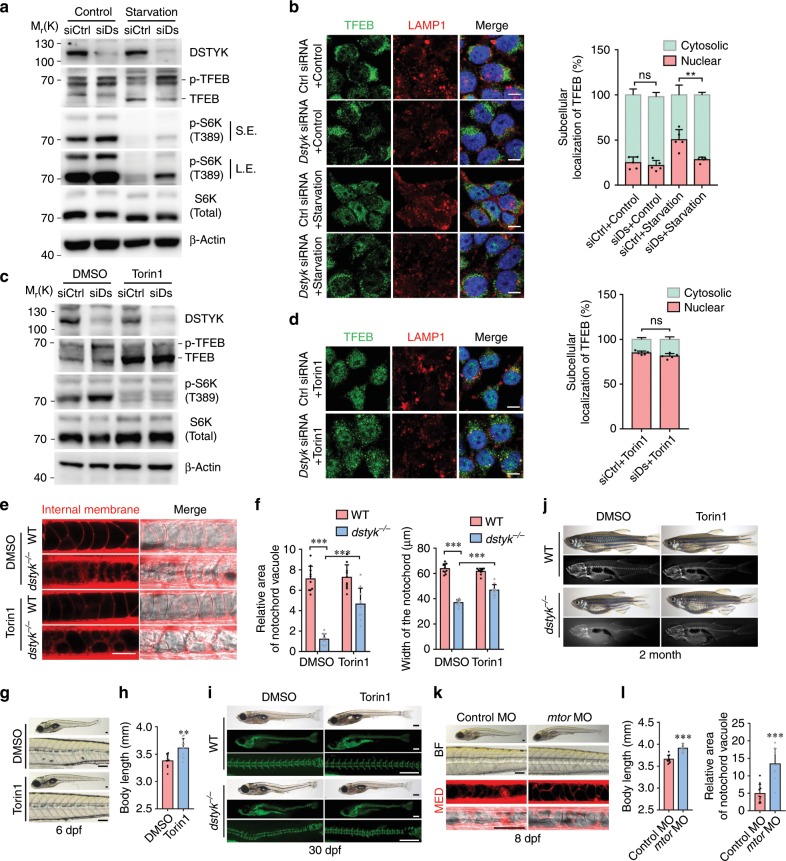
Fig. 9. Dstyk regulates lysosome biogenesis through mTORC1/TFEB pathway.
a, c Immunoblotting of endogenous TFEB, p-TFEB (phosphorylated TFEB) and p-S6K(T389) in COS-7 cells transfected with control siRNA (siCtrl) or Dstyk siRNA (siDs), a cells without starvation (Control) or starved for 2 h. S.E./L.E., Shorter exposure/longer exposure. c cells treated with DMSO or Torin1 (1 µM) for 3 h. b, d Immunofluorescence for TFEB and LAMP1 in COS-7 cells transfected with control siRNA or Dstyk siRNA, b cells without starvation (Control) or starved for 2 h, d cells treated with Torin1 (1 µM) for 3 h. Quantifications of subcellular localization of endogenous TFEB (a percentage of total immunostaining colocalized with nuclear or cytosolic staining) was shown in right panel. n = 5 independent experiments, each experiment counted 10 cells. **p < 0.01. e–j WT and mutant were treated with DMSO or 500 nM Torin1 from 20 to 30 hpf. Vacuole revealed by MED staining (left) at 48 hpf in (e). Quantification of relative area of notochord vacuole (left) (n = 10 independent embryos, each embryo counted 10 vacuoles) and notochord width (right) (n = 10 independent embryos) at 48 hpf in (f). ***p < 0.001. Bright-field images showing the 6 dpf mutants treated with DMSO (top) or Torin1 (bottom) in (g). Quantification of the body length of mutants in (h). n = 10 independent embryos. **p < 0.01. Calcein staining and bright-field images of about 30 dpf WT (top) and dstyk mutants (bottom) in (i). Whole mount and X-rays images of 2-month-old WT (top) and dstyk mutants (bottom) in (j). k Bright-field (top) and MED staining (bottom) images showing the 8 dpf dstyk mutants after injected with control (left) and mtor MO (right). l Quantification of the body length (left) (n = 10 independent embryos) and relative area of notochord vacuole (right) (n = 10 independent embryos, each embryo counted 10 vacuoles) after control and mtor MO injection. ***p < 0.001. p values in (b, d and f) determined by two-way ANOVA test, p values in (h and l) determined by unpaired two-tailed Student’s t-test. Data are presented as mean ± SD. Scale bar represent 10 μm in (b and d), and 50 μm in (e), 100 μm in (g and k), 500 μm in (i). Source data are provided as a Source Data file.