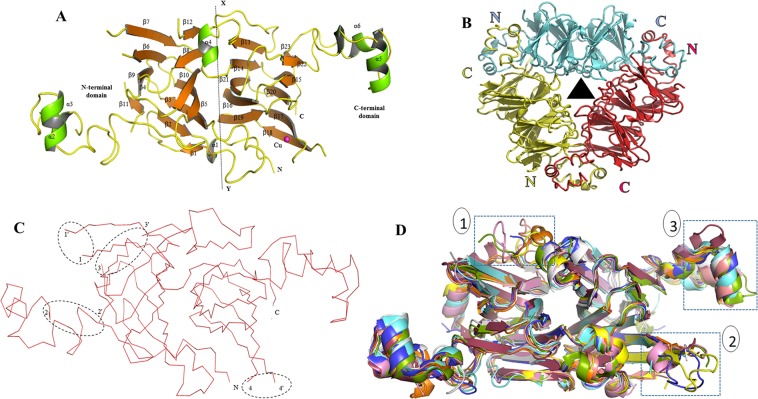
Figure 1.
Crystal structure of Mc7S globulin. (A) The crystallographic asymmetric unit of Mc7S globulin contains a monomer which is represented by a cartoon form. In the monomer, the strands, helixes, and random coils are shown in orange, green, and yellow, respectively. The pseudo-dyad axis of symmetry (X-Y axis) divides the monomer into N and C-terminal domain. Each domain is comprised of a cupin fold. The N and C-terminal ends have been marked as N and C. The β sheets and α-helices have been labelled as per occurrence. The magenta sphere is the Cu ion. (B) The biological Mc7S trimer assembly is formed by interaction of monomers in head-to-tail fashion. The 3 chains of Mc7S is represented in cartoon form in green, pink and purple, respectively. The hydrophobic surface residues are coloured in red. The triangle in the centre represents the axis perpendicular to the three-fold axis of symmetry. (C) Four disordered region of Mc7S (I: 125–127 residues, II: 215–220 residues, III: 225–247 residues, and IV: 325–334 residues) have been circled. The ending residues of the disordered loop is marked by apostrophe (‘). (D) Structural superimposition of vicilin from Solanum melongena SM80.1 (5vf5-golden), pecan (cyan), AraH1 (magenta), Korean pine (green), β-conglycinin (orange), phaseolin (brown), 8S mungbean (purple), Aduzki bean (light red), Jackbean (blue), Capsicum annum Vic_CAPAN (pink) over Mc7S (white). The region of variation is marked by blue dotted box. Box 1 represent the region around disorder loop 3, the box 2 is the copper binding site whereas the box 3 is the C-terminal helical region.