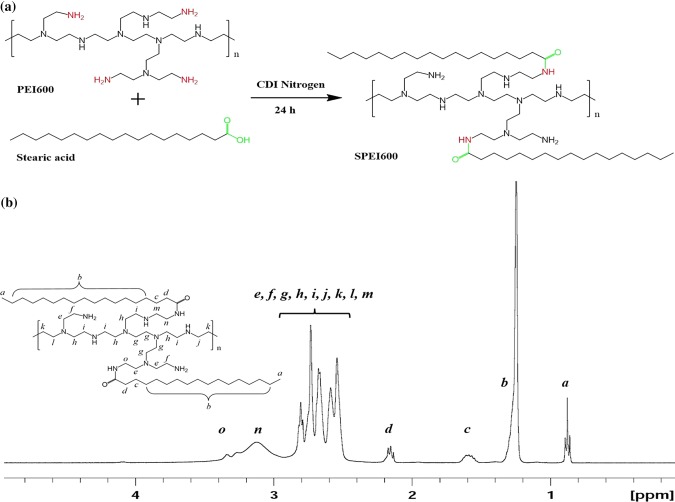
Figure 1.
Synthesis and characterization of SPEI600. (a) Schematic illustration of synthesis of SPEI600. (b) 1H-NMR spectra of SPEI in CDCl3. The chemical shift δ0.9 ppm is assigned to the proton –CH3 on stearic acid backbone (marked for a); δ2.5–2.93 ppm are assigned to the proton –NCH2CH2N– protons of the PEI backbone (marked for e, f, g, h, i, j, k, l and m); δ1.24 ppm is assigned to the proton (CH2)14 on PEI backbone (marked for b); δ1.58 ppm and δ2.13–2.17 ppm are assigned to the proton –COCH2CH2– on stearic acid (marked for c and d); and δ2.93–3.3 ppm are assigned to the proton –CONHCH2– on PEI backbone (marked for n and o).