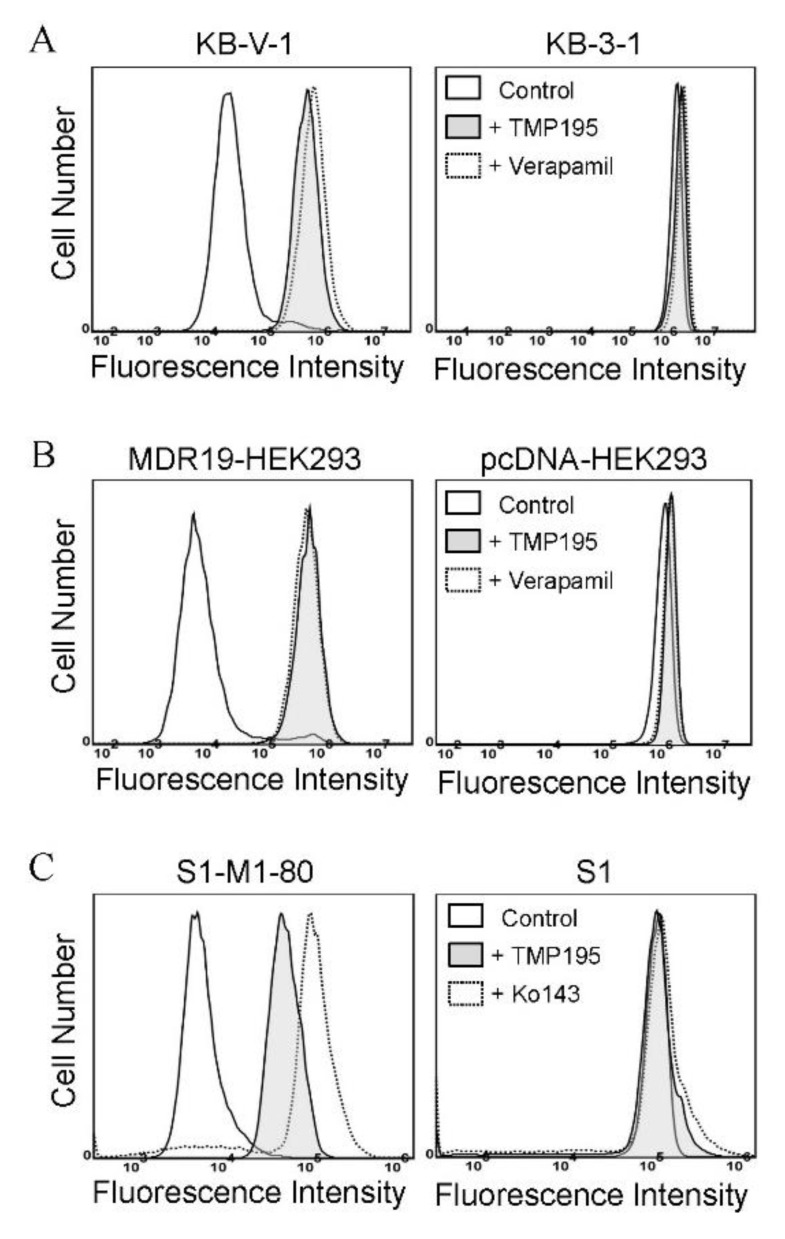
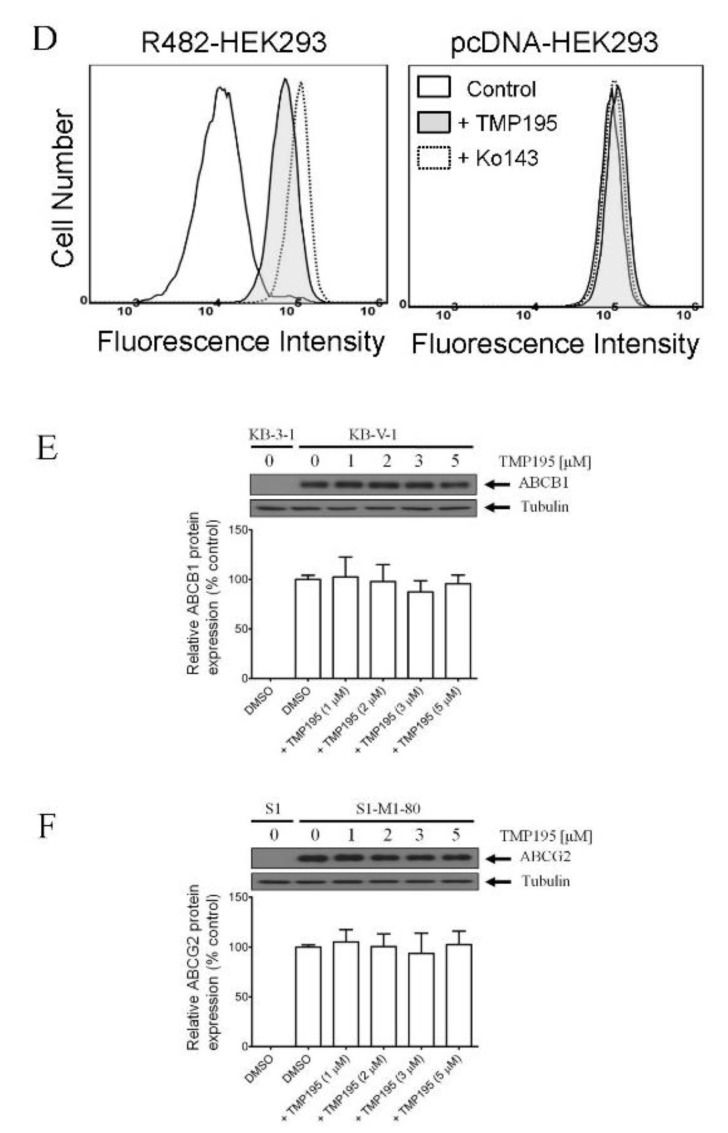
Figure 3.
TMP195 modulates the drug efflux function, but not the protein expression, of ABCB1 and ABCG2 in human multidrug-resistant cancer cells. The effect of TMP195 on ABCB1-mediated transport of calcein-AM, a known substrate of ABCB1, was determined in (A) ABCB1-overexpressing KB-V-1 human epidermal cancer cells and (B) human HEK293 cells transfected with human ABCB1 (MDR19-HEK293), whereas the effect of TMP195 on ABCG2-mediated transport of pheophorbide A (PhA), a known substrate of ABCG2, was determined in (C) ABCG2-overexpressing S1-M1-80 human colon cancer cells and (D) HEK293 cells transfected with human ABCG2 (R482-HEK293). Respective drug-sensitive parental cell lines were used as controls (A–D, right panels). Intracellular fluorescence of calcein (A and B) or PhA (C and D) was measured in cells treated with DMSO (A–D, solid lines), 20 μM of TMP195 (A–D, filled solid lines), 20 μM of verapamil as a positive control for ABCB1 inhibition (A and B, dotted lines), or 1 μM of Ko143 as a positive control for ABCG2 inhibition (C and D, dotted lines) as indicated and analyzed by flow cytometry. Representative histograms of at least three independent experiments are shown. The effect of TMP195 on the protein expression of ABCB1 or ABCG2 was determined by treating (E) KB-V-1 cancer cells or (F) S1-M1-80 cancer cells with increasing concentrations of TMP195 (0–5 μM) as indicated for 72 h before processing for immunoblotting. α-Tubulin was used as an internal loading control. Immunoblot detection (upper panels) and quantification values (lower panels) are presented as mean ± SD calculated from at least three independent experiments.