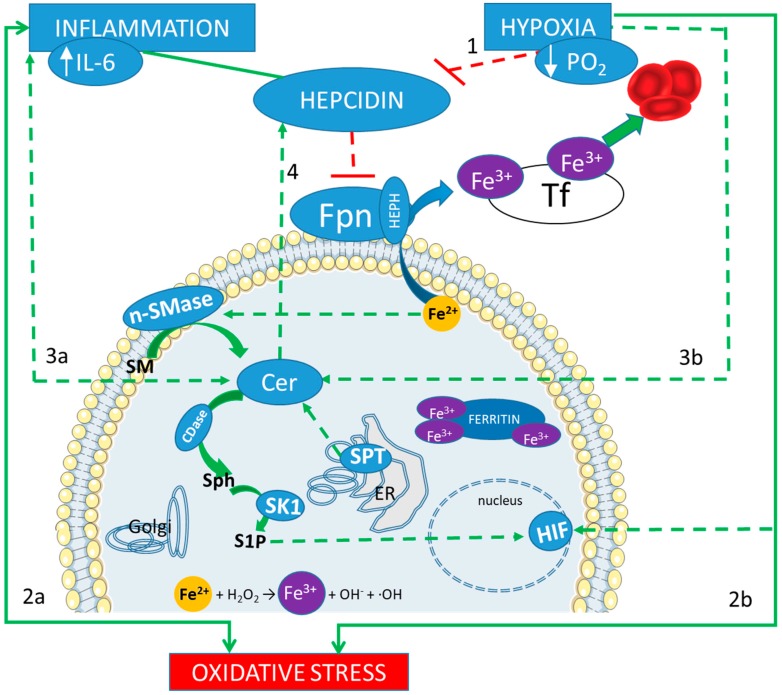
Figure 1.
Iron and sphingolipids interplay in response to inflammation and hypoxia. A correct adaptation to hypoxia results in the inhibition of the regulator peptide hepcidin (line 1). Hepcidin main action is the reduction of the outflow of the intracellular ferrous iron (Fe2+), which is mediated by ferroportin (Fpn). Therefore, if Fpn is less inhibited, iron can be released in the blood stream, bound to the trasporter fransferrin (Tf) in its ferric form (Fe3+), and then reach the bone marrow, to contribute to the hematopoietic response. On the other hand, inflammation induces an increase in hepcidin, which blocks such adaptation. Both inflammation and hypoxia are sources of oxidative stress (lines 2a and 2b). An excess of intracellular iron can be a further source of oxidative stress, through the Fenton reaction (showed at the bottom). Both inflammation and hypoxia increase the production of Ceramide (Cer, lines 3a and 3b) derived by a de novo biosynthetic pathway, mediated by serin palmitoyl transferase (SPT) in the endoplasmic reticulum (ER), and by the hydrolysis of sphingomyelin (SM), mediated by neutral sphingomyelinase (nSMase). Cer accumulation promotes hepcidin expression (line 4) with a consequent increase in intracellular iron content, which, in turn, triggers Cer production (via activation of SM hydrolysis) in a vicious loop. Furthermore, ceramidase (CDase) converts Cer in sphingosine (Sph), which is phosphorylated by sphingosine kinase 1 (SK1) to produce sphingosine 1 phosphate (S1P). S1P acts as an oxygen-independent regulator of HIFs.