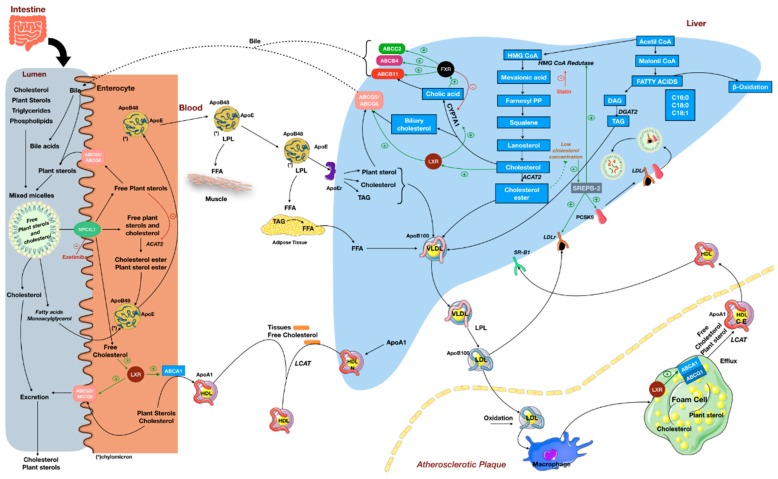
Figure 2.
Plant sterol and cholesterol metabolism. Dietary lipids, biliary cholesterol, and bile acids are incorporated into mixed micelles in the intestinal lumen. Competition between plant sterols and free cholesterol during digestion causes a reduction in cholesterol solubilization and increases cholesterol excretion in feces. Free cholesterol and free plant sterols are absorbed through the NPC1L1 transporter, while other lipids are taken up into the enterocyte by facilitated diffusion at the brush border. Plant sterol absorption is controlled by ABCG5/8, which acts as an efflux pump to export free sterols from enterocytes back into the intestinal lumen. Alternatively, plant sterols may also be packed into lipoproteins in a similar way as cholesterol. After intestinal uptake, dietary and biliary free cholesterol (and some free plant sterols) are normally esterified by ACAT-2, incorporated into chylomicrons, and secreted into the lymph. Unesterified cholesterol is secreted back to the intestinal lumen by ABCG5/8. Chylomicrons reach the circulation and deliver free fatty acids to peripheral tissues through the activity of LPL. Chylomicron remnants undergo hepatic uptake, where they contribute to the formation of VLDL along with cholesterol esters (synthesized through the HMG CoA pathway) and TAG (synthesized through the malonyl-CoA pathway). Once plant sterols reach the liver, they can also be returned to the intestine by ABCG5/8 transporters at the hepatobiliary interface. VLDL particles are secreted into the bloodstream and give rise to LDL particles. LDL distributes cholesterol and plant sterols to tissues and undergoes hepatic uptake through LDL receptors (LDLr). However, LDL particles may infiltrate the endothelial intima where they undergo oxidative and enzymatic modification. Uptake of modified LDL by intima macrophages leads to the formation of foam cells and fatty streaks. The atherosclerotic process may be attenuated by HDL, as it promotes cholesterol efflux from other tissues by LCAT, and also from the macrophages. HDL is recognized by SRB1 receptors in the liver and deliver cholesterol (or plant sterols) for biliary excretion, keeping the “cholesterol reverse transport” cycle. Hepatic cholesterol homeostasis is controlled by several sensors. Decreased levels of cholesterol esters activate SREBP-2, which upregulates HMG CoA reductase, increases LDL receptor (LDLr) expression, and induces expression of PCSK9, that after secretion binds to LDLr on the hepatocyte surface, forming a complex PCSK9–LDLr, which is internalized and undergoes degradation. On the other hand, increased levels of cholesterol in hepatocytes leads to activation of LXR and FXR, which upregulate expression of enzymes and transporters involved with biliary excretion. Activation of LXR in enterocytes leads to luminal excretion of cholesterol and facilitates intestinal HDL synthesis. For details on lipid metabolism see Ref. [41,42,43]. Abbreviations: ABCA1, ATP-binding cassette, subfamily A, member 1; ABCG1, ATP-binding cassette, subfamily G, member 1; ABCG5, ATP-binding cassette, subfamily G, member 5; ABCG8, ATP-binding cassette, subfamily G, member 8; ACAT2, acetyl-CoA acetyltransferase 2; CE, cholesterol ester; CM, chylomicron; DAG, diacylglycerol; DGAT2, diacylglycerol O-acyltransferase 2; FFA, free fatty acids; FXR, farnesoid X receptor; HMG Coa-Reductase, 3-hydroxy-3-methyl-glutaryl-coenzyme A reductase; HDL, high-density lipoprotein; LCAT, lecithin-cholesterol acyltransferase; LPL, lipoprotein lipase; LXR, liver X receptor, NPC1L1, Niemann–Pick C1 like 1; PCSK9, proprotein convertase subtilisin/kexin type 9; SBR1, Scavenger receptor class B member 1; SREBP-2, sterol regulatory element-binding transcription factor 2; TAG, triacylglycerol; and VLDL, very low density lipoprotein.