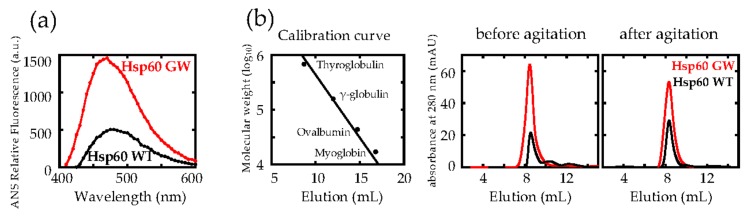
Figure 2.
Comparing the structural characteristics of wild type (WT) and G190W Hsp60. (a) Estimation of protein surface hydrophobicity by ANS binding. (b) Evaluation of Hsp60 quaternary structure using gel-filtration chromatography. Hsp60 samples were applied to a Superdex 200 Increase 10/300 GL column and the relative molecular size was estimated from a curve calibrated with the following molecular weight standards: Thyroglobulin (bovine) 670,000, γ-globulin (bovine) 158,000, Ovalbumin (chicken) 44,000, and Myoglobin (horse) 17,000.