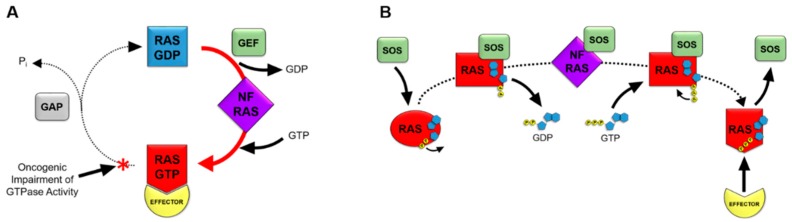
Figure 1.
RAS as a molecular switch in the cell. Figure 1 depicts the mechanisms of RAS nucleotide cycling. RAS proteins cycle from an inactive GDP-bound state to a nucleotide-free (NF) transition state, followed by formation of the active GTP-bound state (A). Guanine nucleotide exchange factors (GEFs) facilitate the exchange of GDP for GTP. The conformation of RAS is dynamic through this exchange, with GTP-bound RAS being in an active conformation that binds effectors containing RAS binding domains. The intrinsic GTPase activity of RAS hydrolyzes GTP to GDP in order to revert back to the inactive state of RAS. GTPase activating proteins (GAPs) promote GTP hydrolysis activity by approximately 1,000-fold. Oncogenic mutations in RAS genes impair the GTPase activity of RAS, resulting in a prevalence of the active, GTP-bound state of RAS in tumor cells. This portion of the cycle prevalent in oncogenic RAS is depicted with a red arrow and asterisk. SOS mediates the exchange of GDP for GTP on RAS proteins (B). SOS binding to RAS results in a conformational shift in RAS, occluding the magnesium cofactor from interacting with the phosphate groups of GDP. The resulting decrease in affinity of RAS for GDP contributes to the release of GDP to form the nucleotide-free (NF) state of RAS. GTP binding to RAS subsequently occurs due to high concentrations of GTP in tumor cells. Initially, only the guanosine and ribose moieties of GTP bind to RAS. Interactions of the gamma phosphate group of GTP with the magnesium cofactor of RAS result in displacement of SOS. The resulting active conformation of GTP-bound RAS can interact with effectors containing RAS binding domains to activate downstream signaling pathways.