Introduction
The 22q11.2 deletion syndrome (22q11.2DS) is the most common chromosomal microdeletion disorder. A broad phenotypical spectrum including congenital cardiac and/or palatal malformations, neuropsychiatric disorders such as intellectual disability, schizophrenia, attention-deficit hyperactivity, autism spectrum, anxiety disorders, and epilepsy exists.1 The reason for this broad phenotypical variability is largely unexplained, but it could be the result of individual or multigenic reduced gene dosage and permissive variants in modifier genes elsewhere in the genome.2 Among movement disorders, Dopa-responsive parkinsonism is the most commonly reported movement disorder.2,3 Myoclonus has rarely been reported and the electrophysiological characterization is lacking.3,4 We report two 22q11.2DS patients with myoclonus of subcortical origin as the presenting movement disorder, with extensive clinical and electrophysiological analysis.
Case presentations
Patient 1
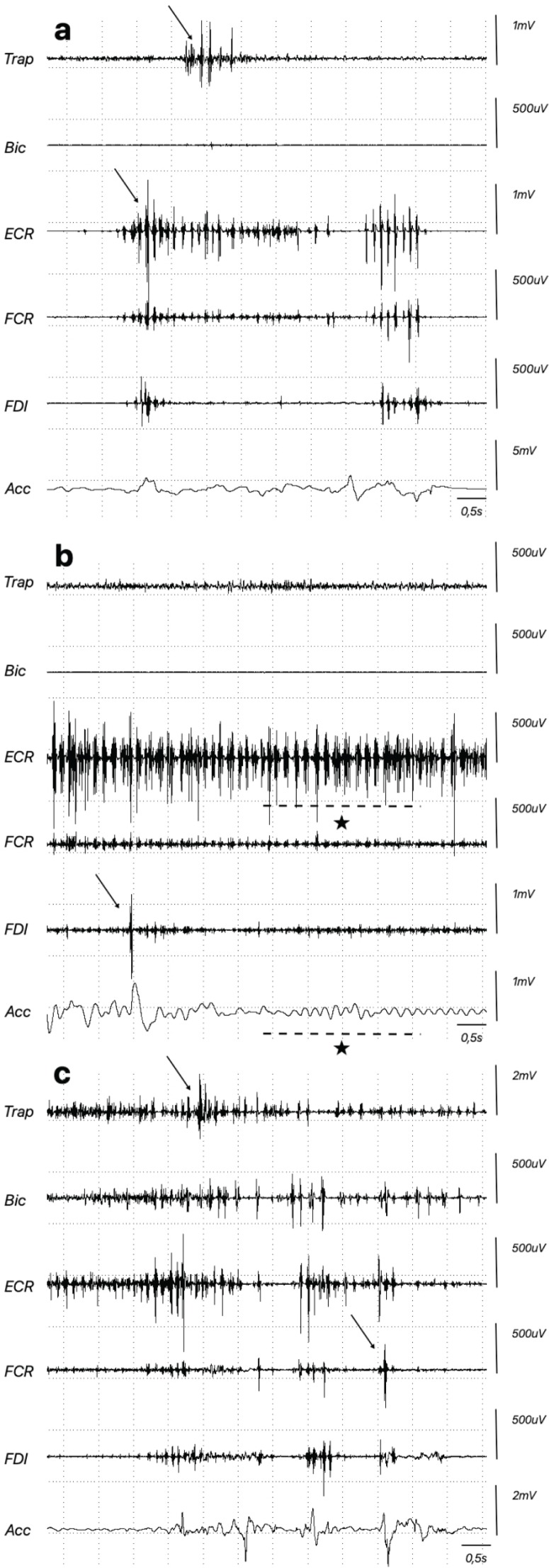
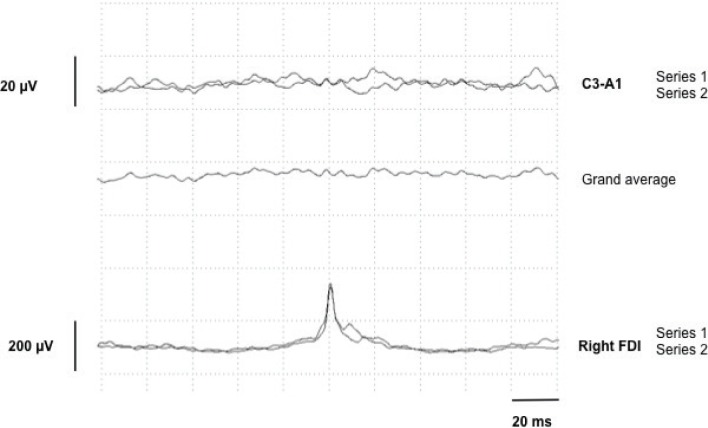
A 41-year-old female (Video 1) presented with childhood-onset upper limb “tremor like” movements that responded to low-dose propranolol and alcohol. Her past medical history was notable for surgical correction of palatal deformation, recurrent respiratory infections, and mild psychomotor developmental delay. Clinical examination demonstrated high frequency upper limb myoclonic jerks present during rest, posture and worsening with action. Dystonic posturing was noted in both arms in the outstretched position and in the right hand during writing. Myoclonic jerks in the lower limbs were also present at rest. She had a long face, telecanthus, thin upper lip, and smooth philtrum. Electrophysiological examination of the right upper limb revealed myoclonic bursts occurring at rest, posture and action, ranging between 38 and 108ms (mean of 73ms, calculated on 32 myoclonic bursts) (Figure 1). There was no premyoclonic potential on jerk-locked EEG–EMG back averaging (JLBA) (Figure 2) and C-reflex was absent. Brain magnetic resonance imaging (MRI) showed non-specific subcortical T2 hyperintensities. Cardiac echography and routine blood analysis were normal. Dopamine transporter (DaT) scan was normal and levodopa challenge did not result in any benefit. Chromosomal microarray demonstrated a hemizygous pathogenic 2.5 Mb deletion of the 22q11.2 chromosomal region. This chromosomal deletion is the classical deletion between LCR22-A and LCR22-D and contains more than 70 genes.
Figure 1.
Myoclonus Recorded on Surface EMG in the Right Upper Limb and Accelerometer (Acc) Attached to the Right Index Finger. Some arrhythmic myoclonic jerks are shown with an arrow. Note the tremor-like postural irregular rhythmic myoclonus (*) in (B). (A) Rest; (B) extension of wrist; (C) drawing a spiral. Trap, trapezius; Bic, biceps; ECR, extensor carpi radialis; FCR, flexor carpi radials; FDI, first dorsal interosseous.
Figure 2.
Jerk-Locked EEG–EMG Back Averaging (JLBA) in Patient 1 with Two Series Comprising a Total of 200 Myoclonic Bursts Registered in the Right First Dorsal Interosseous (FDI). There is no evidence of a pre-myoclonic potential on EEG recordings.
Video 1.
Patient in a Sitting Position with Myoclonic Jerks of the Upper and Lower Limbs during Rest, while Maintaining Posture, and during Action. The myoclonic jerks interfered with holding a glass of water and pouring water from one glass into another. Dystonic posturing of both hands in an outstretched and flexed posture of the arms can be seen. During writing with the right hand, dystonic posturing of the first and second fingers and hyperextension of the wrist is also seen. The following facial features can be observed: thin upper lip, smooth philtrum, telecanthus, and long face.
Patient 2
A 27-year-old woman (Video 2) was diagnosed with 22q11.2 deletion aged 4 years in the context of cardiomyopathy and immunodeficiency. Psychomotor development was normal. At age 16, she was referred to the movement disorders clinic with a 2-year history of upper-limb tremor. Clinical examination demonstrated upper-limb myoclonic jerks at rest in the right forearm and during outstretched and flexed posture of the left arm. The jerks worsened with actions such as drinking from a glass and drawing a spiral. Associated dystonic posturing was noted in the left hand and in the right hand when writing. She had a slight triangular face, thin upper lip, and plump nose. Electrophysiological examination confirmed diffuse myoclonus with myoclonic bursts present at rest, while maintaining posture, and during action (range 18–96 ms); no premyoclonic potential on JLBA; and no C-reflex. Brain MRI, DaT scan, lumbar puncture, and blood analysis reports were normal. Treatment with propranolol and piracetam proved moderately effective.
Video 2.
Patient in a Sitting Position with Myoclonic Jerks in the Right Upper Limb during Rest and in the Left Upper Limb during Posture. Myoclonic jerks get worsened with various actions. With the arms held up and elbows in a flexed posture, dystonic posturing can be seen in the left hand. When writing, dystonic posturing of the right thumb and index finger as well as hyperextension of right wrist can be seen together with associated dystonic posturing of the left hand. Drawing a spiral also shows dystonic posturing of the left hand. No remarkable findings are noted during walking. A triangular face, smooth philtrum, and plump nose are the prominent facial features.
Discussion
We report two patients who presented at a young age with myoclonus (Figure 1B) combined with dystonia in the context of 22q11.2DS. In the absence of electrophysiological analysis, the multiple rest and action jerks could have been mistaken for tremor. Electrophysiological findings suggested a subcortical origin of the myoclonus: muscle burst durations above 70 ms,5 absence of either C-reflex or premyoclonic potential on jerk-locked EEG–EMG back averaging. The presence of dysmorphic facial features and learning difficulties in the first patient motivated micro-array analysis. The second patient presented to the movement clinic with new onset myoclonus and mild dystonic posturing on a background of 22q11.2DS. Development was normal, but discrete facial features were also present. These two observations expand the spectrum of movement disorders in 22q11.2DS.
Previous reports have described early-onset levodopa-responsive parkinsonism and patients with parkinsonian features without a formal diagnosis of Parkinson’s disease (PD) in 22q11.2DS.2,6 To our knowledge, only one other case with primary myoclonus in the absence of epilepsy has been reported, describing a female patient with myoclonus of head, trunk, and limbs since the age of 5.4 Other rare cases have been associated with myoclonic epilepsy, suggestive of a cortical origin, or myoclonus induced by neuroleptic treatment.3,7,8
Interestingly, the COMT gene of the catechol-O-methyltransferase enzyme, responsible for dopamine degradation, is located in the classical 22q11.2 deletion region.9,10 Elevated levels of presynaptic dopamine have previously been observed on functional neuroimaging studies in young adult patients with 22q11.2DS without a formal diagnosis of PD.11 It has been hypothesized that hyperdopaminergic autotoxicity could lead to PD.11 Whether this hyperdopaminergic autotoxicity plays a role in determining the phenotype of our patients is unclear taking into account the complex genetic disorder. As with other pathologies, myoclonus could be missed or erroneously called “tremor” (see Lagarde et al.12). Myoclonus could also be secondary to a wide range of causes in the 22q11 deletion syndrome: hypocalcemia, thyroid dysfunction, neuroleptic-induced myoclonus, or levodopa treatment because of parkinsonism.3 In the work by Baralle,4 myoclonus was described in two 22q11DS patients without an obvious cause despite extensive explorations. In clinical practice, it is often difficult to distinguish between symptoms due to 22q11DS and comorbid disorders. Moving forward, careful clinical observation for the emergence of parkinsonism in both cases will be needed. It is noteworthy that among the deleted genes no other gene is known to be directly or indirectly involved in movement disorders and therefore to contribute to genotype–phenotype correlations.
In summary, subcortical myoclonus combined with dystonia may be considered as part of the movement disorder phenotypic spectrum in 22q11.2DS. As such, the association of subcortical myoclonus with dysmorphic facial features warrants micro-array analysis to investigate possible 22q11.2DS.
Acknowledgments
The authors thank both patients for their participation in the study.
Footnotes
Citation: Van Iseghem V, McGovern E, Apartis E, Keren B, Vidailhet M, Roze E, et al. Subcortical myoclonus and associated dystonia in 22q11.2 deletion syndrome. Tremor Other Hyperkinet Mov. 2020; 10. doi: 10.7916/tohm.v0.729
Editor: Elan D. Louis, Yale University, USA
Funding: None.
Financial Disclosures: V. Van Iseghem received a clinical fellowship grant from EAN (European Academy of Neurology). E. Mc Govern received travel grants from Elivie and EAN. Marie Vidailhet received travel grants from MDS (Movement Disorders Society) and EAN. E. Roze received support for research from Merz-Pharma, Orkyn, Aguettant, Elivie, Ipsen, Fondation Desmarest, AMADYS, Fonds de Dotation Brou de Laurière, and Agence Nationale de la Recherche; he has served on scientific advisory boards for Orkyn, Aguettant, Merz-Pharma; has received honoraria for speeches from Orkyn, Aguettant, Merz-Pharma, Everpharma, and International Parkinson and Movement Disorders Society; has received travel grants from Vitalair, Aguettant, Merz-Pharma, Ipsen, Merck, Orkyn, Elivie, Dystonia medical Research Foundation, International Parkinson and Movement Disorders Society, European Academy of Neurology, and International Association of Parkinsonism and Related Disorders. B. Degos received support for research from Orkyn, Elivie, and Leadiant; he has received honoraria for speeches from Ipsen and has received a travel grant from Merz-Pharma.
Conflicts of Interest: The authors report no conflicts of interest.
Ethics Statement: All patients who appear on the video have provided written informed consent and authorization for videotaping and publication of the videotape.
Authors’ contribution
V. Van Iseghem and E. McGovern were responsible for the conception, organization, and execution of the research project and also were responsible for writing of the article. E. Apartis was responsible for the execution of the research project and review and critical analysis of the article. M. Vidailhet and B. Keren were responsible for review and critical analysis of the article. E. Roze and B. Degos were responsible for the conception and organization of the research project and review and critical analysis of the article.
References
- 1.McDonald-McGinn DM, Sullivan KE, Marino B, Philip N, Swillen A, Vorstman JAS, et al. . 22q11.2 deletion syndrome. Nat Rev Dis Prim 2015;1:15071. doi: 10.1038/nrdp.2015.71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dufournet B, Nguyen K, Charles P, Grabli D, Jacquette A, Borg M, et al. . Parkinson’s disease associated with 22q11.2 deletion: clinical characteristics and response to treatment. Rev Neurol (Paris) 2017;173(6):406–410. doi: 10.1016/j.neurol.2017.03.021 [DOI] [PubMed] [Google Scholar]
- 3.Boot E, Butcher NJ, van Amelsvoort TA, Lang AE, Marras C, Pondal M, et al. . Movement disorders and other motor abnormalities in adults with 22q11.2 deletion syndrome. Am J Med Genet Part A 2015;167(3):639–645. doi: 10.1002/ajmg.a.36928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baralle D. Myoclonic movement disorder associated with microdeletion of chromosome 22q11. J Neurol Neurosurg Psychiatry 2002;73(5):600–601. doi: 10.1136/jnnp.73.5.600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caviness JN, Brown P. Myoclonus: current concepts and recent advances. Lancet Neurol 2004;3(10):598–607. doi: 10.1016/S1474-4422(04)00880-4 [DOI] [PubMed] [Google Scholar]
- 6.Boot E, Bassett AS, Marras C. 22q11.2 Deletion syndrome-associated Parkinson’s disease. Mov Disord Clin Pract 2019;6(1):11–16. doi: 10.1002/mdc3.12687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Strehlow V, Swinkels MEM, Thomas RH, Rapps N, Syrbe S, Dorn T, et al. . Generalized epilepsy and myoclonic seizures in 22q11.2 deletion syndrome. Mol Syndromol 2016;7(4):239–246. doi: 10.1159/000448445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lemke JR, Beck-Wödl S, Zankl A, Riegel M, Krämer G, Dorn T. Juvenile myoclonic epilepsy with photosensitivity in a female with velocardiofacial syndrome (del(22)(q11.2)) – causal relationship or coincidence? Seizure 2009;18(9):660–663. doi: 10.1016/J.SEIZURE.2009.07.008 [DOI] [PubMed] [Google Scholar]
- 9.Boot E, Booij J, Abeling N, Meijer J, da Silva Alves F, Zinkstok J, et al. . Dopamine metabolism in adults with 22q11 deletion syndrome, with and without schizophrenia – relationship with COMT Val108/158 Met polymorphism, gender and symptomatology. J Psychopharmacol 2011;25(7):888–895. doi: 10.1177/0269881111400644 [DOI] [PubMed] [Google Scholar]
- 10.Boot E, Booij J, Zinkstok J, Abeling N, De Haan L, Baas F, et al. . Disrupted dopaminergic neurotransmission in 22q11 deletion syndrome. Neuropsychopharmacology 2008;33:1252–1258. doi: 10.1038/sj.npp.1301508 [DOI] [PubMed] [Google Scholar]
- 11.Butcher NJ, Marras C, Pondal M, Rusjan P, Boot E, Christopher L, et al. . Neuroimaging and clinical features in adults with a 22q11.2 deletion at risk of Parkinson’s disease. Brain 2017;140(5):1371–1383. doi: 10.1093/brain/awx053 [DOI] [PubMed] [Google Scholar]
- 12.Lagarde J, Roze E, Apartis E, Pothalil D, Sedel F, Couvert P, et al. . Myoclonus and dystonia in cerebrotendinous xanthomatosis. Mov Disord 2012;27(14):1805–1810. doi: 10.1002/mds.25206 [DOI] [PubMed] [Google Scholar]