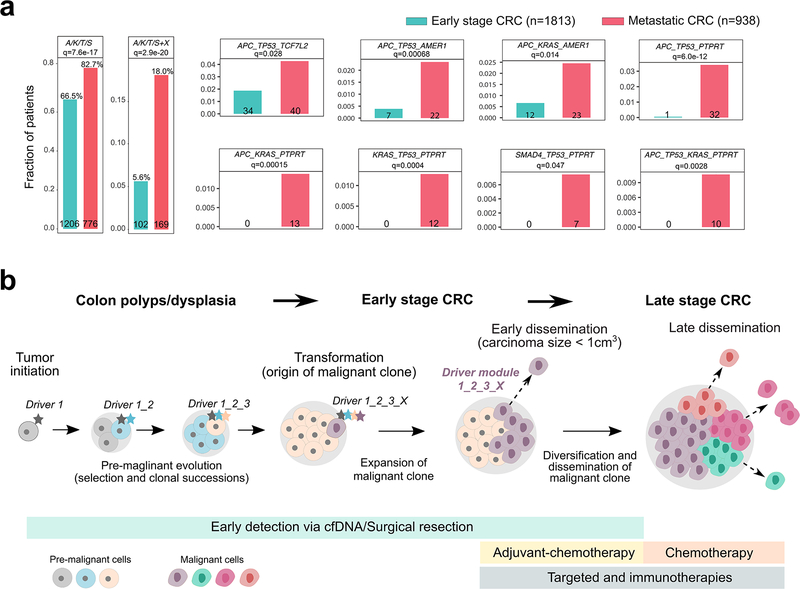
Figure 6. Enrichment of early driver gene modules in mCRC and clinical implications of early dissemination.
(a) The enrichment of canonical ‘core’ CRC driver genes (APC, KRAS, TP53 or SMAD4; A, K, T or S) plus recurrent mutations in candidate drivers (AMER1, ATM, BRAF, PIK3CA, PTPRT or TCF7L2) identified in the mCRC cohort was evaluated in an independent cohort of 2,751 CRC patients. The combined barplots (left) illustrate the overall frequency of the ‘core’ module alone or with an additional candidate driver (‘X’) in early stage versus metastatic CRCs. Individual barplots indicate the frequency of specific ‘modules’. Q-values are based on two-sided Fisher’s exact tests with Benjamini–Hochberg adjustment. (b) Three stages of CRC progression are outlined: pre-malignancy (between initiation and transformation), early-stage (between transformation and dissemination) and late-stage (after dissemination). A set of potential interventions to prevent cancer mortality targets each stage: for pre-malignant lesions, resection (after detection via colonoscopy or possibly cell free DNA; cfDNA); for early-stage CRC, surgical resection and possibly adjuvant chemotherapy; and for late-stage CRC, chemotherapy and/or targeted/immune therapies. Given the high rate (80% here) of early dissemination, prior to clinical detectability of the early-stage CRC, detection and resection of pre-malignant lesions will have the greatest impact on preventing cancer mortality. For tumors that undergo dissemination prior to clinical detectability, surgical resection alone, even of a small tumor, cannot prevent metastasis. Once the early-stage tumor is discovered, newly defined metastatic modules (panel a) may inform patient stratification to aid the directed use of adjuvant chemotherapy.