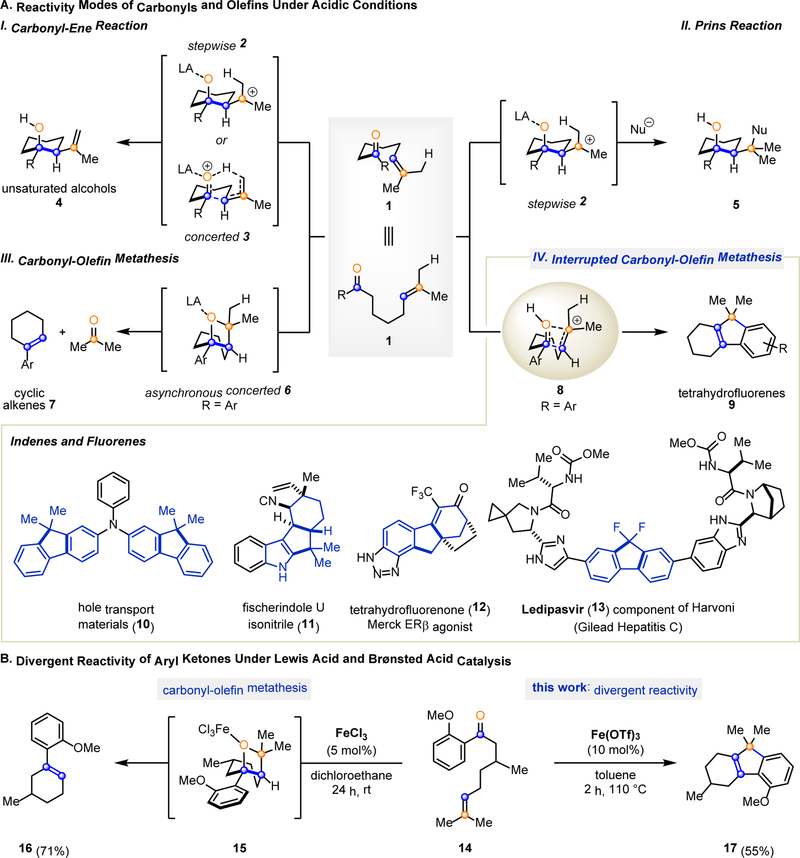
Fig. 1. Chemistry of Carbonyls and Olefins.
A. Fundamental acid-catalyzed reactivity modes between carbonyls and olefins. (I) Carbonyl-ene reaction; (II) Prins reaction; (III) Carbonyl-olefin metathesis; (IV) This work: interrupted carbonyl-olefin metathesis; importance of the products accessible in this reactivity mode is showcased below. LA, Lewis acid. B. Complementary reactivity modes of aryl ketone 14 are accessible depending on the choice of iron(III)-derived Lewis acid catalyst. The use of FeCl3 leads to the formation of the carbonyl-olefin metathesis product 16, whereas Fe(OTf)3 results in tetrahydrofluorene 17.