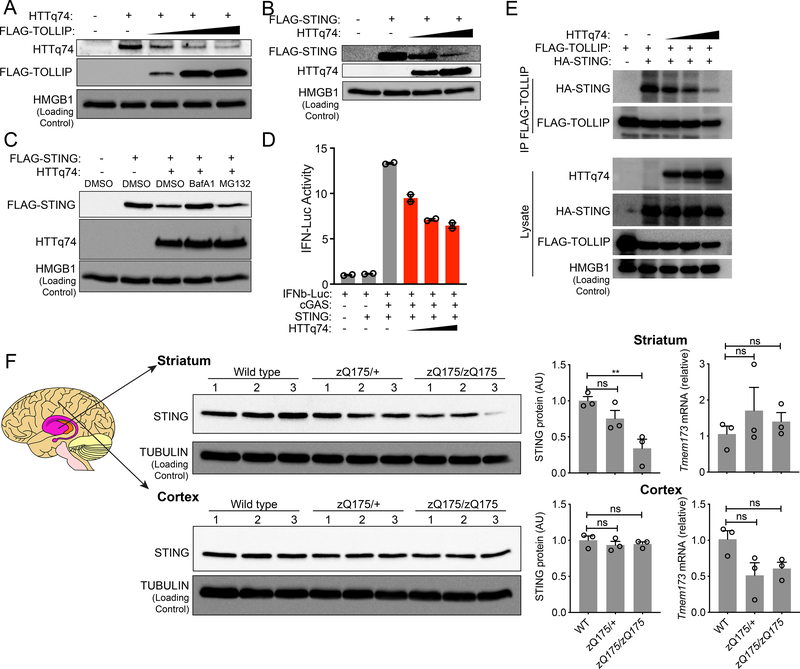
Figure 4: PolyQ protein disrupts TOLLIP:STING interaction and results in STING protein turnover in vitro and in vivo.
(A) Immunoblot analysis of HEK293T cells transfected with the same amount HTTq74 plasmid and increasing amount of FLAG-TOLLIP plasmids (as indicated on top).
(B) Immunoblot analysis of HEK293T cells transfected with the same amount Flag-STING plasmid and increasing amount of HTTq74 plasmids (as indicated on top).
(C) Immunoblot analysis of STING and HTTq74 co-expression treated overnight with MG-132 (5 μM) and BafA1 (0.5 μM).
(D) IFNβ-Luciferase assay of cGAS-STING activation after dose titration of HTTq74 protein. A representative experiment with 2 biological replicates is showing. Experiments were repeated twice.
(E) Co-immunoprecipitation of STING and TOLLIP in HEK293T cells. HEK293T cells were transfected with indicated plasmids (top) in the presence of BafA1 (0.5 μM), and 24 h later anti-FLAG antibody was used to pull down FLAG-TOLLIP. HA-STING co-IP was analyzed by immunoblot. Note, BafA1 was used to prevent STING degradation caused by polyQ protein.
(F) STING protein and mRNA analysis in zQ175 knock-in mouse brain tissues. Wild type, zQ175/+ and zQ175/zQ175 mouse brain striatum and cortex were freshly dissected and processed for immunoblot and qRT-PCR analysis. n=3 mice per group. STING protein blots are showing on the left, densitometry quantitation of STING/TUBULIN band ratio is showing in the middle, and STING mRNA level (normalized to GAPDH) is showing on the right. For panel F, **, P < 0.01; n.s., not significant. Error bars represent the SEM. Unpaired Student’s t test (2-sided).
Data are representative of at least two independent experiments.