Fig. 2.
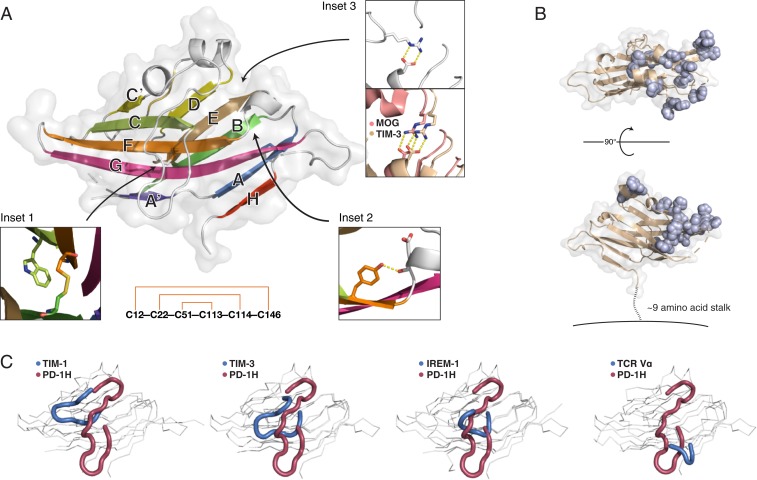
PD-1H crystal structure reveals a striking surface organization of histidines and a long, circuitous CC′ loop. (A) The human PD-1H extracellular domain structure in cartoon and surface representation. β-Strands are labeled according to standard IgV nomenclature, but including an additional unique H-strand. (Inset 1) Zoomed view of the canonical B–F disulfide packing against the conserved tryptophan to form the hydrophobic core between β-sheets. (Inset 2) Zoomed view of the “Tyrosine-corner” that is present in PD-1H and is common in IgV domains. (Inset 3) PD-1H (Left) lacks the conserved arginine-aspartate salt bridge in IgV proteins, such as MOG (salmon, Right) or TIM-3 (wheat, Right), instead using an arginine from the CC′ loop to form an alternative salt bridge at the same location. (Lower): “Outside–in” pattern of disulfide bonding connectivity in the PD-1H extracellular domain. (B) The structure of PD-1H in 2 views as a cartoon (wheat) with a transparent surface. All histidine residues are displayed in sphere representation (light blue) to emphasize their spatial clustering within one-half of the PD-1H ECD, proximal to the CDR-equivalent region. A short stalk of ∼9 amino acids (dashed line) connects the ECD to the membrane (solid line). (C) Comparison of CC′ loop regions from PD-1H (red) and other IgV-like domains (blue), including those with long CC′ loops.