Significance
A central issue in robust patterning mediated by morphogens is how to buffer variability in morphogen distribution between embryos. We previously described a class of mechanisms that buffers variability through “distal pinning,” a global feedback mechanism that modulates the spread of the gradient and terminates only when morphogen values at a distal position reach some given, fixed level. We have previously shown in Drosophila embryonic dorso-ventral patterning that the secreted WntD protein modulates Toll pathway activation. We have altered the wntD enhancer and demonstrate a global contraction of the Toll gradient. The demonstration of a direct link between the enhancer properties of the modulator gene and the morphogen activation profile establishes a molecular mechanism for buffering variability in morphogen distribution.
Keywords: morphogen gradients, embryogenesis, integral feedback, Drosophila, Toll signaling
Abstract
Buffering variability in morphogen distribution is essential for reproducible patterning. A theoretically proposed class of mechanisms, termed “distal pinning,” achieves robustness by combining local sensing of morphogen levels with global modulation of gradient spread. Here, we demonstrate a critical role for morphogen sensing by a gene enhancer, which ultimately determines the final global distribution of the morphogen and enables reproducible patterning. Specifically, we show that, while the pattern of Toll activation in the early Drosophila embryo is robust to gene dosage of its locally produced regulator, WntD, it is sensitive to a single-nucleotide change in the wntD enhancer. Thus, enhancer properties of locally produced WntD directly impinge on the global morphogen profile.
The profile of morphogen gradients determines the resulting arrays of gene expression that govern embryonic body pattern formation. Buffering variability in morphogen distribution between individual embryos is essential to achieve reproducible patterning. We previously described a general class of mechanisms that buffers variability through “distal pinning,” a global feedback mechanism that continuously modulates the spread of the gradient and concludes only when morphogen value at a distal position reaches some given, fixed level (1–3). Since the feedback acts globally, pinning the morphogen level at one point effectively determines the distribution throughout the field. Thus, the morphogen gradient can withstand fluctuations in the different parameters controlling its establishment. The gradient, however, remains sensitive to the parameters defining its “pinning value,” namely the value at the distal position, the attainment of which terminates the feedback.
We recently suggested that a distal pinning mechanism buffers fluctuations in patterning of the dorso-ventral (DV) axis in early Drosophila embryos. Here, the global feedback is exerted by a feedback inhibitor, WntD, the expression of which is restricted to a specific region of the embryo and further depends on the patterning signal itself: the level of nuclear-localized Dorsal protein (4). While wntD is expressed locally in the syncytial embryo, it encodes a secreted protein that diffuses readily within the extracellular milieu. WntD is therefore produced as long as nuclear Dorsal is higher than some threshold, defined by the sensitivity of the wntD enhancer. Since WntD narrows down the nuclear Dorsal gradient, wntD expression eventually stops. Thus, at steady state, the levels of nuclear Dorsal, specifically at the region expressing wntD, are “pinned” to the threshold level defined by the wntD enhancer. This model therefore predicts, perhaps counterintuitively, that changing the copy number of wntD will have no effect on the final gradient, but that modulating wntD expression by altering its enhancer will change the global distribution of nuclear Dorsal and therefore perturb patterning. We previously showed that DV axis patterning is indeed robust to changes in wntD copy number (4). We next wanted to examine whether changing the endogenous wntD enhancer properties would modulate the pattern.
Results
Rationale.
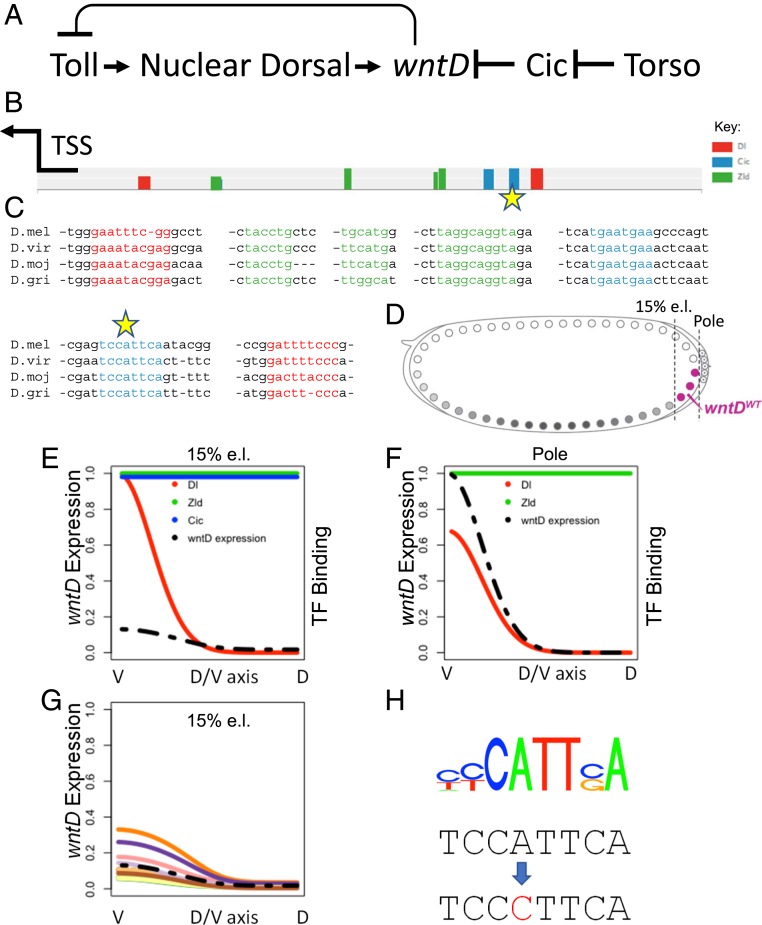
Nuclear entry of the transcription factor Dorsal in the early Drosophila embryo depends on the Toll pathway. The Toll receptor is activated in a graded manner, instructed by a gradient of its ligand Spaetzle (Spz) (5, 6). Expression of the feedback-regulated pathway inhibitor wntD is restricted to the termini of the embryo following Torso-induced ERK phosphorylation and removal of the maternally provided ubiquitous transcriptional repressor Capicua (Cic) (Fig. 1A) (7). While wntD is expressed locally in the syncytial embryo, it encodes a secreted protein that diffuses readily within the extracellular milieu and acts globally. In addition to binding sites for Cic, the wntD enhancer also has binding sites for Dorsal that tune its expression according to the level of Toll activation within each embryo (Fig. 1 A–C). WntD binds the Toll receptor and blocks binding of the activating ligand Spz (4). Thus, the levels of WntD tune Toll signaling by regulating the number of available receptors.
Fig. 1.
Modeling the wntD enhancer. (A) wntD expression is triggered by Toll signaling that leads to nuclear targeting of Dorsal and is repressed by Cic binding. Secreted WntD protein in turn attenuates the activation of Toll. (B) Predicted binding sites for the TFs Dorsal (red), Zld (green), and Cic (blue) within the wntD enhancer (a 500-bp segment upstream of the wntD transcriptional start site), using known motifs for these TFs. The heights of the bars represent predicted site strengths. The arrow marks the transcription-start site (TSS) of wntD. (C) Sequence alignment for the binding sites marked in B shows that the sites are highly conserved among evolutionarily distant species (D. melanogaster, D. virilis, D. mojavensis, and D. grimshawi). (D) Schematic of the syncytial D. melanogaster embryo and wntD expression domain (magenta). We modeled the expression profile of wntD along the DV axis in 2 different locations, the posterior pole and 15% egg length (e.l.), marked by the vertical dashed lines. The modeling assumes a cylindrical embryo, i.e., the existence of a DV axis at each anterior–posterior position from 0 e.l. to 100% e.l. (E and F) Predicted binding levels of the wild-type wntD enhancer by the 3 TFs (colored solid lines), and the resulting wntD expression profile (black dashed line) along the DV axis, are shown at 15% e.l. (E) and at the posterior pole (F). Expression levels and TF binding are on a relative scale with maximum expression or binding along the DV axis, across both anterior–posterior positions, being assigned a value of 1. (G) Predicted DV expression profile of wntD at 15% e.l. with the selected Cic-binding mutation, using an ensemble of models, predicting an elevated expression. Each line represents the prediction of a cluster of models in the ensemble. The dashed line is the wild-type expression, as shown in E. (H) The selected mutation changes the high-specificity A at position 4 of the distal Cic motif (position −358 of the enhancer) to a C (marked by stars in B and C).
WntD is expected to accumulate, and thereby narrow down the Toll activation gradient, until the level of Toll signaling falls below the threshold for wntD induction. The wntD expression domain that is defined by Torso activation and Cic phosphorylation displays intermediate levels of Toll signaling. It follows that the enhancer landscape of wntD is directly related to the final global shape of the Toll activation gradient.
To check this prediction, we sought to alter the sensitivity of the wntD enhancer and examine the impact on the final pattern of the Toll activation gradient.
Compact Organization of the wntD Enhancer.
To examine whether changing the activation threshold of wntD will modify the global Toll signaling profile, it was necessary to define which sequences in the wntD enhancer regulate its activation threshold. We used the thermodynamics-based model generator GEMSTAT (8, 9) to quantitatively describe the combinatorial logic of transcription factor (TF) binding sites within the wntD enhancer. The GEMSTAT models identify binding sites for one or more TFs in the enhancer and use the strengths of these sites, as well as cellular concentrations of the TFs, to predict the expression level driven by that enhancer. Putative binding sites for the TFs Dorsal, Zelda (Zld), and Capicua (Cic), which are highly conserved in diverse Drosophila species, were identified within the wntD enhancer (Fig. 1C). A subset of the models made predictions consistent with expression data for the wild-type wntD enhancer, i.e., little or no detectable expression in the trunk region and a DV patterned expression at the poles (Fig. 1 E and F; see also ref. 4).
We then used this subset of models to predict the effect of single-nucleotide mutations in the enhancer, which will alter wntD expression levels with minimal disruption to enhancer structure. Specifically, we looked for a way to partially relieve the repression of the wntD enhancer by Cic in order to make wntD expression more responsive to activation by Dorsal signaling. We selected such a mutation, located at a key position in the more distal of the 2 Cic-binding sites, which is predicted to strongly diminish the binding to this site. The models vary in their exact assessment of the consequences of this mutation, but the majority agree in predicting de-repression of wntD expression in the trunk region due to weakening of the Cic site (Fig. 1 G and H).
Modulation of One Cic-Binding Site Leads to Broader Expression of wntD.
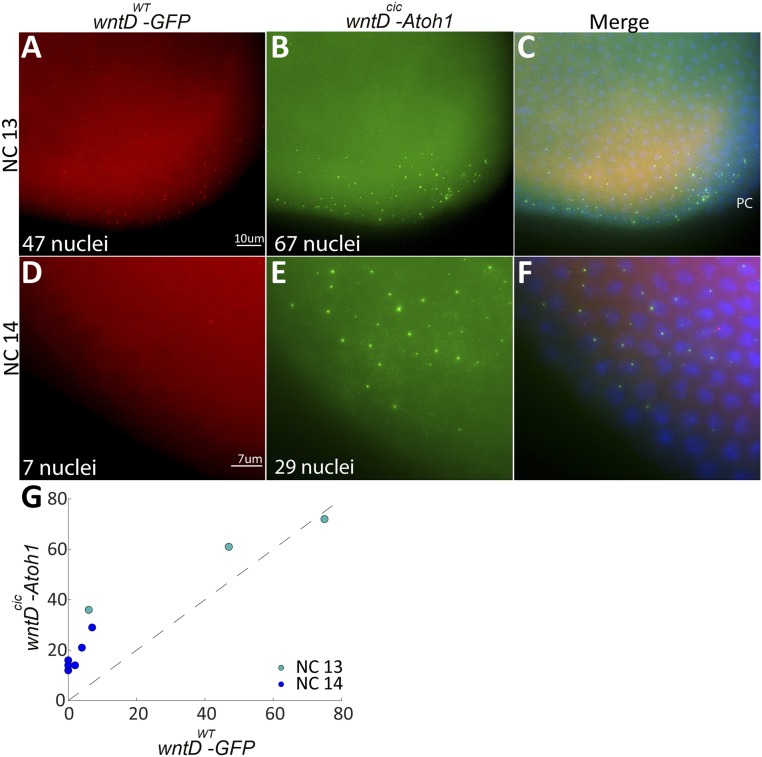
To test if modulation of the distal Cic site within the wntD enhancer will indeed expand the normal wntD expression domain, we generated 2 transgenic reporter fly lines harboring distinct transcriptional outputs. In one reporter construct, the intact wntD enhancer (wntDWT) drives expression of green fluorescent protein (GFP), while in the second construct, the modified wntD enhancer (wntDcic) drives expression of the murine gene Atoh1 (10). For accurate comparison, we followed expression of both reporters within the same embryo, collecting embryos at nuclear division cycles 12 through 14. Furthermore, since we wanted to monitor active transcription, rather than accumulation of transcripts over time, we utilized single-molecule RNA fluorescence in situ hybridization (FISH) for detection. Active transcription is apparent as a strong, focused spot of hybridization within the nucleus, distinct from the dispersed signal of accumulated transcripts in the cytoplasm (Fig. 2).
Fig. 2.
Modulation of a Cic-binding site expands wntD expression. Modeling suggested that alteration of a Cic-binding site would expand wntD expression. smFISH was used to follow active reporter transcription and compare the expression domains of the wild-type wntD enhancer (wntDWT) driving GFP expression (A and D, red) and a modified wntDcic enhancer driving mouse Atoh1 (B and E, green) within staged single embryos. (C and F) The merged images, along with DAPI staining (blue) to visualize the nuclei. PC, pole cells. (A–C) At NC 13, both constructs are expressed at the posterior pole of the embryo, but expression of Atoh1 via wntDcic is broader and encompasses more nuclei. (D–F) By NC 14, expression of GFP via wntDwt is almost diminished, indicating that steady state has been reached, while wntDcic still drives Atoh1 expression in a significant number of nuclei. (G) Compilation of data from multiple embryos shows that the number of nuclei expressing the wntDcic-Atoh1 construct is always higher. Each point on the plot represents an embryo fixed at a specific time point in the embryo. The x axis represents the number of wntD-expressing nuclei via the wild-type enhancer while the y axis represents the number of wntD-expressing nuclei via the mutant enhancer. The dashed line is the x = y boundary; therefore, for points above the dashed line, the number of expressing nuclei is greater for the mutant enhancer and vice versa. Two trends are observed: 1) All but one dot are well above the dashed line, indicating a greater amount of expressing nuclei for the mutant enhancer throughout NCs 13 and 14, and 2) The dark blue points depicting NC 14 all show a smaller number of expressing nuclei than the green points displaying NC 13, indicating a decrease in the amount of expressing nuclei between NCs 13 and 14 for both WT and mutant enhancers.
Cic protein is maternally provided and uniformly distributed within the embryo (11). Torso signaling at the termini triggers ERK, leading to phosphorylation and nuclear export of Cic. Thus, the wntD enhancer is accessible to activation by Dorsal only at the termini. wntD transcription at the anterior terminus is delayed since Bicoid competes for phosphorylation by ERK (12). At early time points, when both reporters are expressed, the modified wntD enhancer is expected to be less sensitive to repression by Cic and therefore to drive expression in a comparatively larger number of nuclei, occupying a broader domain. Later on, wntDcic should continue to drive expression at a time when the endogenous enhancer is nearly silent.
We validated both of these predictions in embryos. At nuclear cycle (NC) 13 both reporters are expressed, but the expression of wntDcic-Atoh1 encompasses more nuclei (Fig. 2 A and B). By NC 14, expression of GFP driven by the wntDWT enhancer is restricted to a small number of nuclei, while wntDcic is still driving expression of Atoh1 in a significant number of nuclei (Fig. 2 D and E). Compilation of data from multiple embryos shows that during these nuclear cycles the number of nuclei expressing Atoh1 via wntDcic is always larger (Fig. 2G).
Gastrulation of a Reduced Mesoderm Domain upon Modulation of a Cic-Binding Site.
Since the wntDcic enhancer reporter is capable of driving a broader and prolonged expression compared to the wild-type enhancer, we wanted to monitor the biological outcomes, following a similar alteration to the expression profile of endogenous wntD. We used standard CRISPR methodologies to modify the endogenous wntD enhancer, so that the distal Cic-binding site is compromised similar to the wntDcic reporter. Embryos harboring this modification are homozygous viable and will be referred to as wntDcic embryos. Our model predicts that the lower threshold for wntDcic expression would lead to prolonged expression and give rise to a global reduction in the steady-state distribution of Toll activation gradient. We therefore monitored the distribution of Toll activation in wild-type and wntDcic embryos using different outputs.
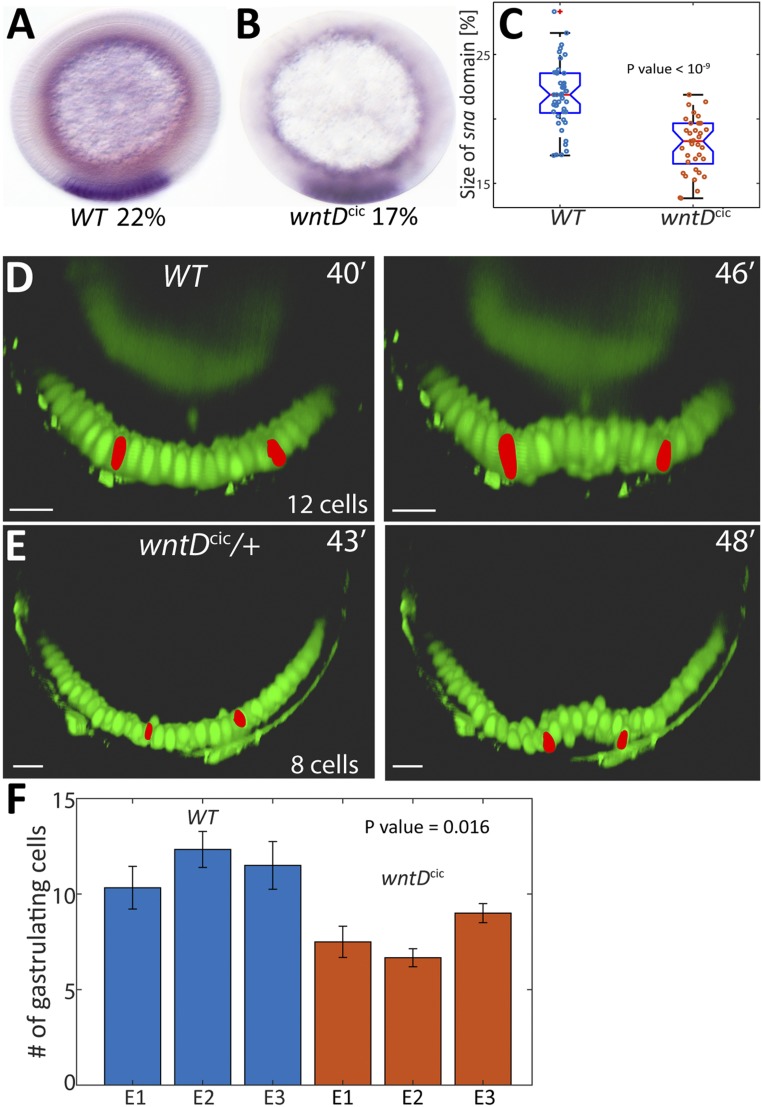
One measure of the activation profile is the expression domain of snail (sna), a Dorsal-target gene, which typically occupies 22% of the embryo circumference, centered on the ventral midline, in wild-type embryos (13). In homozygous wntDcic embryos, the sna expression domain was reduced to 18% of the circumference (Fig. 3 A–C). Another measure for the Toll-activation pattern is the number of cells undergoing invagination into the ventral furrow at the onset of gastrulation. Expansion of the Toll profile was shown to lead to a broader front of gastrulating cells (14, 15). We thus wanted to examine if the change in wntD enhancer properties will give rise to the predicted reduction in the number of gastrulating cells, due to a narrowing of the Toll activation profile. Toward this end, heterozygous wntDcic embryos carrying a Dorsal-GFP reporter (16) were monitored by live imaging using light sheet fluorescence microscopy. Our earlier work demonstrated that altering the number of wild-type wntD copies changes the dynamics but leads to a similar steady-state Toll activation profile (4). We therefore reasoned that a single copy of the modified wntDcic gene would be sufficient to alter the steady-state profile.
Fig. 3.
Expanded wntD expression alters the global Toll activation profile. (A and B) The circumferential proportion of the domain expressing sna (purple ventral stripe) was monitored as a measure for the width of the Toll pathway activation profile. (C) The size of the sna domain, measured in multiple embryos, was consistently higher in wt embryos than in wntDcic embryos. Box plots of percent of sna domain sizes: Boxed area defines 25 to 75 percentile and the whiskers extend to the most extreme points not considered outliers. Mean is marked by red line. Note that at NC 14, when prominent sna expression is induced, expression of wntDcic did not reach termination and steady state (Fig. 2E). Thus, monitoring sna expression is an under-estimate of the capacity of excess WntD levels to influence and restrict Toll pathway activation under these conditions. (D and E) Analysis and comparison of the number of cells undergoing ventral furrowing, monitored by live imaging of Dorsal-GFP (green), which localizes to ventral nuclei, in wt and wntDcic/+ embryos. Nuclei at the lateral edges of the ventral furrow (red) served as guideposts for the extent of the gastrulating domain. (F) Quantitation of 3 embryos of each genotype demonstrates that the size of the invagination domain is consistently smaller in wntDcic/+ embryos (P value indicated on plots C and F was calculated using unpaired t test). Error bars reflect differences in measurement between 3 anterior-posterior locations within each embryo. (Scale bars, 20 μm.)
Once gastrulation ensues, the extent of the ventrally furrowing domain—the future mesoderm—can be defined by identifying the most lateral nuclei that alter their orientation. While it is difficult to count the number of gastrulating nuclei once furrow formation initiates, tracing these edge nuclei back in time allows us to score the number of nuclei between them at the earlier monolayer blastoderm stage. Using this approach, we find that the ventrally furrowing domain is indeed significantly and reproducibly smaller in wntDcic/+ heterozygous embryos, dropping from 12 to 8 gastrulating cells (Fig. 3 D–F and SI Appendix, Fig. S1).
Discussion
We examined the consequences of altering the enhancer of the wntD gene. We previously suggested that WntD function generates an integral-feedback loop that buffers the global distribution of the Toll-activation gradient (4). Our model assigns a critical role for the enhancer because it determines the fixed point at which transcription of wntD will stop and the Toll activation gradient will reach steady state. The model predicts that the gradient is stable to alternation in wntD copy number, since each copy is buffered by the very same integral-feedback loop mediated by WntD. In contrast, the gradient is highly sensitive to changes in the wntD enhancer that alter the binding sensitivity to its regulators. We previously confirmed that changes in wntD gene dosage are indeed of no consequence to the final global pattern (4). Here, we showed that modulation of the endogenous wntD enhancer indeed affects the global Toll-activation pattern.
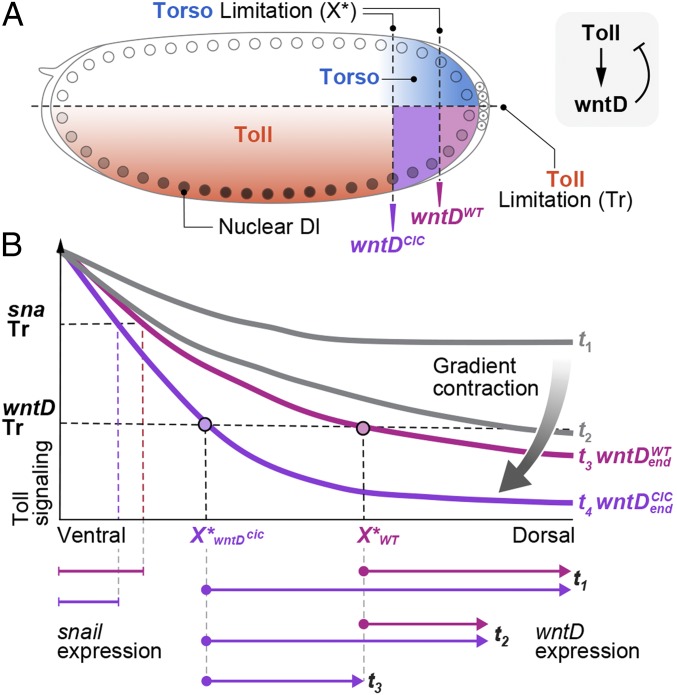
Specifically, we predicted and verified that a single-nucleotide change in the wntD enhancer will modify its expression properties, making it less sensitive to repression and thus allowing wntD to be expressed in a broader domain and for longer time periods. The consequence is that higher levels of WntD protein are uniformly distributed and accumulate within the perivitelline fluid surrounding the embryo, leading to a global reduction in the steady-state profile of Toll activation. Within the framework of our integral-feedback model, lower levels of nuclear Dorsal are sufficient for activating expression of the modified wntD enhancer. Accordingly, its expression will terminate and steady state will be reached, only when the gradient will be further narrowed to reach this new and lower pinned level. Indeed, by monitoring the zygotic output Toll signaling, we showed that the snail expression domain, defining the future mesoderm, became narrower. In addition, the number of cells undergoing ventral furrowing was reduced (summarized in Fig. 4).
Fig. 4.
wntD enhancer determines the global Toll activation profile. (A) In the syncytial Drosophila embryo, the Toll activation gradient is orthogonal to Torso signaling at the termini. Induction of wntD expression requires both signals and hence is confined to the termini, where Torso signaling removes the transcriptional repression by Cic. Due to the geometry of the embryo, this domain is exposed to intermediate levels of Toll signaling and displays intermediary levels of Dorsal-nuclear localization, accordingly. Modulation of 1 of the 2 Cic-binding sites in the wntD enhancer gave rise to broader and prolonged expression of wntD. (B) Early embryos (t1) display an excess of Toll activation manifested by a broad activation profile. This leads to local induction of wntD expression only in the restricted wntD expression zone which is defined by X > X*. X* is determined by the wntD enhancer and appears in magenta for wntDWT and purple for wntDcic. The size of the wntD expression domain is determined by Toll-signaling levels, which must be above the threshold (Tr) in order to induce wntD expression. The wntD expression zone is indicated by the arrows below the plot for wntDWT (magenta) and wntDcic embryos (purple). The secreted WntD protein is broadly distributed, giving rise to a global contraction of Toll signaling. During the reduction in Toll signaling, the expression of wntD is decreased (t2) and is terminated at t3 (wntDWT, magenta), when Toll-signaling levels reach steady state and fall below the threshold of wntDWT induction. wntDcic embryos (purple) continue to express wntD at this point, leading to further contraction of Toll signaling at steady state (t4). The expression domains of target genes such as sna are reduced accordingly.
The enhancer of a gene encoding a secreted modulator plays a critical role with broad implications for achieving robust patterning. Since modulator expression is regulated by the morphogen, it will terminate only when the morphogen profile reaches steady state at a fixed point. This point is determined by the enhancer properties of the modulator and will thus define the final global distribution of the morphogen activation gradient (3). Under this regime, the patterning system can tolerate variability in the initial activation profile, as long as there is sufficient time for production and diffusion of the secreted modulator.
The general concept, whereby enhancers of secreted feedback-regulators play a critical role in shaping patterning gradients, can be executed by diverse molecular mechanisms. In the Drosophila wing imaginal disc, distribution of the Dpp morphogen is modulated by the secreted protein Pentagone (Pent) (17). This protein facilitates a longer diffusion range of Dpp by triggering endocytosis of the coreceptors (18). Since pent expression is repressed by Dpp signaling, expression will terminate when Dpp signaling levels at the edge of the field reach the fixed point for pent repression. This mechanism allows to scale the distribution of the Dpp gradient with the size of the wing disc (2, 19). Recently, the secreted Scube2 protein was shown to play a similar role during zebrafish neural-tube development in buffering the distribution of the Sonic Hedgehog (Shh) gradient. Due to repression by Shh signaling, Scube2 is expressed only at the edge of the gradient and facilitates the distribution of the morphogen (20). WntD provides another example for the Distal pinning paradigm. In this case, the signals are reversed relative to Pent or Scube2: WntD constricts the activation gradient and is activated by morphogen signaling (4). The restricted wntD expression to lower values of the morphogen gradient, which is necessary to avoid complete morphogen signaling shutdown, is achieved by the Torso pathway that confines wntD expression to the embryo termini (Fig. 4A).
Secreted proteins that modulate the global morphogen profile represent critical and potent external regulatory knobs that are not part of the core signaling cascade. Fine-tuning their expression properties by enhancer modification may thus impinge on the global morphogen distribution profile without altering the structure of proteins that constitute the primary signaling pathway. During evolution of new species, a single change in the enhancer that drives expression of the secreted modulator may be sufficient to alter the global distribution of a morphogen, and hence the size of the field that will be patterned.
Methods
Bioinformatics.
The ensemble of models taken from ref. 9 consists of a set of models, each one being a setting of free parameters of GEMSTAT that accurately predict the DV expression pattern of the wild-type ind enhancer using its sequence and the TF concentration profiles of the TFs Dorsal, Zld, and Cic as well as Vnd and Sna. The model relies upon predetermined binding motifs (position weight matrices) of the TFs to identify and quantify binding site strengths in the enhancer. Here, we used an ensemble of models, trained on an enhancer of the gene intermediate neuroblasts defective (ind) in our previous work (9), and applied it to the wntD enhancer. Binding motifs for Dorsal, Zld, and Cic were obtained from FlyFactorSurvey (21). We used DNaseI hypersensitivity data from stage 5 embryos (22) to identify a 500-bp segment of the wntD enhancer that exhibits high levels of DNA accessibility and harbors a cluster of TF-binding sites for Dorsal, Zld, and Cic. We separately predicted wntD expression across the DV axis at 15% egg length (same position as that modeled in ref. 9) and at the poles; Zld was assumed to have a uniform concentration profile along the DV axis in both cases, Cic was assumed to be uniformly expressed in the trunk but absent at the poles, and Dorsal was assumed to have a gradient along the DV axis, with the ventral peak expression being higher in the trunk region than at the poles (Fig. 1 E and F). We selected the subset of models, the predictions of which for the wild-type wntD enhancer were within a small root-mean-square-deviation (RMSE) of the known expression readout in the trunk and poles. We constructed a probability distribution over these models following the procedure in ref. 9 and computed the predicted effect of each single-nucleotide mutation in the enhancer as the average, over this distribution of the RMSE between predicted DV expression profiles with and without the mutation. An “A” to a “C” mutation at position −358 in the 500-bp enhancer, which targets a high-specificity position in a Cic-binding site and had among the largest predicted effects of any single mutation, was chosen for further study. The wntD enhancer sequence used in the analysis is the following: ATGATGAACCGGGTCAGCACACTTATATAGCCTGCAAATCCCAAGCCAGGGCGCCCTCCTGGGGCCGGCCCGTGGGAATTTCGGGCCTGCTCAAAAAACCGGAAATTTGCCGTTTTCCACTTGGAAATTTTGCATGGGCAGGGGGTAGGAACTCCCGGCAATGGACGGGTACAAAAACCCACTGGCAGCCCGAGACGCAATTGCGGAGCAGCCCAGTTTCCTGGTTGACTACCTGCTCTCGTCCTGCGCCGGCGGAGGTGAAGGATCCGCCTTGCTGCGAGCAAGTTTCCCACGCTTAGGCAGGTAGAGCCGTAAACGGCACCCGACGTGCTCATGAATGAAGCCCAGTCGAGTCCATTCAATACGGCCGGATTTTCCCGGACTCACACTGCACAACATCAATGCCCGATACGGGGACGGGTTTGTTTGGGTTTTGGACTGGTCAAGCCAATTATATAACAAAACATATGACCAACAGTATATACACGTATAATCTGGGA. The mutation changes the Cic site TCCATTCA to TCCCTTCA.
Engineering of Fly Strains.
The wntDwt > GFP; wntDcic > Atoh1 strain used for single-molecule FISH (smFISH) harbors 2 reporter transgenes. The wntDwt > GFP reporter was generated by synthesizing a DNA construct which included the 1,162-bp region upstream of the wntD transcription start site followed by the coding region of superfolder GFP (23). wntDcic> Atoh1 was generated by replacing the wild-type enhancer and GFP sequences with a synthesized sequence which included the wntDcic enhancer and the coding sequence of murine Atoh1 (10). Both constructs were inserted into UASp-attB plasmids and integrated into the chromosomal AttP40 and AttP2 sites, respectively.
The wntDcic strain was generated by CRISPR-induced replacement of the entire wntD gene with a sequence that contains an A > C point mutation at position 4 of the distal cic-binding site (Fig. 1H). We used the following oligonucleotide pairs to generate the relevant guide RNAs: CTTCGAAACCACCTGTAGCTAAAAC, AAACGTTTTAGCTACAGGTGGTTTC; and CTTCGAAGTCCTGTCTGCGTAGCAC;AAACGTGCTACGCAGACAGGACTTC.
To follow the ventrally invaginating cells during gastrulation, we used Sco/CyO; dorsal-GFP/TM3, Sb flies (16), a strain in which the ventral nuclei are labeled by a Dorsal-GFP fusion protein.
smFISH.
A Stellaris RNA FISH probe set for the sfGFP gene was designed by Stellaris Probe Designer and labeled with Quasar670 from LGC Biosearch Technologies. The probe set for the murine Atoh1 gene, labeled with Texas Red, was a gift from the S. Itzkovitz laboratory, Weizmann Institute, Rehovot, Israel. smFISH was carried out as in Rahimi et al. (15). Embryos carrying both reporter constructs were collected for 1 h after egg laying followed by a 2-h incubation, fixed for 25 min in 4% formaldehyde, washed in methanol, and kept at −20 °C. Next-day embryos were washed in methanol and then in ethanol, rocked in 90% Xylene, 10% ethanol for 1 h followed by postfixation and then incubated 6 min with Proteinase K and postfixed again. Embryos were transferred gradually to 10% formamide (deionized) (FA) in 2× Saline Sodium-Citrate buffer (SSC) + 10 μg/mL single-stranded DNA (ssDNA) preheated to 37 °C and prehybridized for 30 min at 37 °C. Hybridization buffer included 10% FA, 10% Dextran, 2 mg/mL bovine serum albumin, ribonucleoside vanadyl complex (RVC), and ssDNA + transfer RNA in 2× SSC, containing the probe set (1 ng/μL) (24). Hybridization was carried out overnight at 37 °C. Next day the embryos were shaken gently and incubated for another 30 min. Embryos were washed twice for 30 min at 37 °C with 10% FA in 2× SSC + 10 μg/mL ssDNA and gradually transferred to phosphate-buffered saline/0.5% Tween and mounted with Vectashield + DAPI Mounting Medium (Vector Laboratories Inc.). Fluorescence was visualized with a Nikon Eclipse Ti2 microscope and analyzed by the TransQuant script as was previously described (25).
Snail In Situ Hybridization and Expression Domain Quantitation.
In situ hybridization for sna was carried out as in Rahimi et al. (4). Fixation of the embryos was carried out in 4% paraformaldehyde, and probe hybridization was carried out at 55 °C. The expression domain was detected via a digoxigenin (DIG)-labeled DNA probe, followed by anti–DIG-alkaline phosphatase-conjugated antibody and substrate detection (Roche), and visualized following cross-sectioning in the middle of the embryo using a tungsten needle. Quantitation of the relative size of the sna domain of the full-embryo circumference was carried out using a MATLAB script that manually identifies the expression domain and calculates the angle generated from the center divided by 360.
Light Sheet Fluorescence Microscopy and Quantitation of Ventral Furrowing.
Embryos were imaged using a Light Sheet Z.1 microscope (Zeiss Ltd.) equipped with 2 sCMOS cameras PCO-Edge, 10× excitation objectives, and Light Sheet Z.1 detection optics 20×/1.0 (water immersion). The embryos were collected and dechorionated, and up to 4 embryos were sequentially mounted perpendicularly into a glass capillary in a 1% low-melting agarose solution. Imaging was performed using dual-side illumination, zoom ×0.8 (GFP excitation: 488 nm; emission/detection: bandpass (BP) 505 to 545; Texas Red excitation: 561 nm, emission/detection: BP 575 to 615 nm).
Optical cross-sections were generated using the Imaris program. The edges of the furrow were defined by marking the 2 lateral-most nuclei that alter their angle with respect to the embryo circumference upon gastrulation. The time-lapse movie was then played backward to an earlier phase of NC 14, when the nuclei are still in a monolayer, in order to accurately count the number of furrowed nuclei.
All data are contained in the manuscript text and SI Appendix.
Supplementary Material
Acknowledgments
We thank S. Ben-Moshe, K. Bahar Halpern, and S. Itzkovitz for advice on smFISH and Y. Addadi and O. Golani for help in acquisition and analysis of light sheet fluorescence microscopy images. Imaging using the light sheet fluorescence microscope was made possible thanks to the de Picciotto-Lesser Cell Observatory founded in memory of Wolf and Ruth Lesser. We thank members of the B.-Z.S. and N.B. laboratories for fruitful discussions. This work was supported in part by NIH Grants R01 GM114341 and R35 GM131819 (to S.S.); United States-Israel Binational Science Foundation Grant 2017055 (to N.B.); United States-Israel Binational Science Foundation Grant 2015063 (to E.D.S. and B.-Z.S.); and a research grant from the Henry Chanoch Krenter Institute for Biomedical Imaging and Genomics (to B.-Z.S.). N.B. is the incumbent of the Lorna Greenberg Scherzer Professorial Chair, and B.-Z.S. is the incumbent of the Hilda and Cecil Lewis Professorial Chair in Molecular Genetics.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.1918268117/-/DCSupplemental.
References
- 1.Ben-Zvi D., Barkai N., Scaling of morphogen gradients by an expansion-repression integral feedback control. Proc. Natl. Acad. Sci. U.S.A. 107, 6924–6929 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ben-Zvi D., Pyrowolakis G., Barkai N., Shilo B. Z., Expansion-repression mechanism for scaling the Dpp activation gradient in Drosophila wing imaginal discs. Curr. Biol. 21, 1391–1396 (2011). [DOI] [PubMed] [Google Scholar]
- 3.Shilo B. Z., Barkai N., Buffering global variability of morphogen gradients. Dev. Cell 40, 429–438 (2017). [DOI] [PubMed] [Google Scholar]
- 4.Rahimi N., et al. , A WntD-dependent integral feedback loop attenuates variability in Drosophila Toll signaling. Dev. Cell 36, 401–414 (2016). [DOI] [PubMed] [Google Scholar]
- 5.Haskel-Ittah M., et al. , Self-organized shuttling: Generating sharp dorsoventral polarity in the early Drosophila embryo. Cell 150, 1016–1028 (2012). [DOI] [PubMed] [Google Scholar]
- 6.Stein D., Roth S., Vogelsang E., Nüsslein-Volhard C., The polarity of the dorsoventral axis in the Drosophila embryo is defined by an extracellular signal. Cell 65, 725–735 (1991). [DOI] [PubMed] [Google Scholar]
- 7.Helman A., et al. , RTK signaling modulates the Dorsal gradient. Development 139, 3032–3039 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.He X., Samee M. A., Blatti C., Sinha S., Thermodynamics-based models of transcriptional regulation by enhancers: The roles of synergistic activation, cooperative binding and short-range repression. PLoS Comput. Biol. 6, e1000935 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khajouei F., Sinha S., An information theoretic treatment of sequence-to-expression modeling. PLoS Comput. Biol. 14, e1006459 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tomic G., et al. , Phospho-regulation of ATOH1 is required for plasticity of secretory progenitors and tissue regeneration. Cell Stem Cell 23, 436–443.e7 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lim B., et al. , Kinetics of gene derepression by ERK signaling. Proc. Natl. Acad. Sci. U.S.A. 110, 10330–10335 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim Y., et al. , Gene regulation by MAPK substrate competition. Dev. Cell 20, 880–887 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rusch J., Levine M., Threshold responses to the dorsal regulatory gradient and the subdivision of primary tissue territories in the Drosophila embryo. Curr. Opin. Genet. Dev. 6, 416–423 (1996). [DOI] [PubMed] [Google Scholar]
- 14.Heer N. C., et al. , Actomyosin-based tissue folding requires a multicellular myosin gradient. Development 144, 1876–1886 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rahimi N., et al. , Dynamics of Spaetzle morphogen shuttling in the Drosophila embryo shapes gastrulation patterning. Development 146, dev181487 (2019). [DOI] [PubMed] [Google Scholar]
- 16.DeLotto R., DeLotto Y., Steward R., Lippincott-Schwartz J., Nucleocytoplasmic shuttling mediates the dynamic maintenance of nuclear Dorsal levels during Drosophila embryogenesis. Development 134, 4233–4241 (2007). [DOI] [PubMed] [Google Scholar]
- 17.Vuilleumier R., et al. , Control of Dpp morphogen signalling by a secreted feedback regulator. Nat. Cell Biol. 12, 611–617 (2010). [DOI] [PubMed] [Google Scholar]
- 18.Norman M., Vuilleumier R., Springhorn A., Gawlik J., Pyrowolakis G., Pentagone internalises glypicans to fine-tune multiple signalling pathways. eLife 5, e13301 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hamaratoglu F., de Lachapelle A. M., Pyrowolakis G., Bergmann S., Affolter M., Dpp signaling activity requires Pentagone to scale with tissue size in the growing Drosophila wing imaginal disc. PLoS Biol. 9, e1001182 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Collins Z. M., Ishimatsu K., Tsai T. Y. C., Megason M. G., A Scube2-Shh feedback loop links morphogen release to morphogen signaling to enable scale invariant patterning of the ventral neural tube. bioRxiv: 10.1101/469239 (13 November 2018). [Google Scholar]
- 21.Zhu L. J., et al. , FlyFactorSurvey: A database of Drosophila transcription factor binding specificities determined using the bacterial one-hybrid system. Nucleic Acids Res. 39, D111–D117 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li X. Y., et al. , The role of chromatin accessibility in directing the widespread, overlapping patterns of Drosophila transcription factor binding. Genome Biol. 12, R34 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pédelacq J. D., Cabantous S., Tran T., Terwilliger T. C., Waldo G. S., Engineering and characterization of a superfolder green fluorescent protein. Nat. Biotechnol. 24, 79–88 (2006). [DOI] [PubMed] [Google Scholar]
- 24.Trcek T., Lionnet T., Shroff H., Lehmann R., mRNA quantification using single-molecule FISH in Drosophila embryos. Nat. Protoc. 12, 1326–1348 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bahar Halpern K., Itzkovitz S., Single molecule approaches for quantifying transcription and degradation rates in intact mammalian tissues. Methods 98, 134–142 (2016). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.