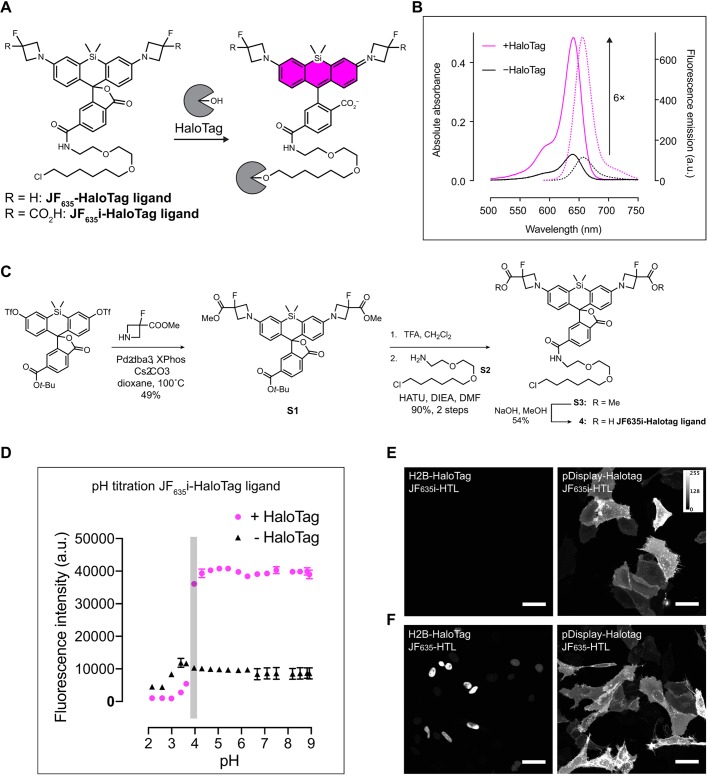
Fig. 2.
JF635i-HaloTag ligand synthesis. (A) Chemical structure of JF635-HaloTag ligand before and after binding to HaloTag. (B) The absorption and emission spectra of JF635i-HaloTag ligand were measured in the medium as the free ligand and when bound to an excess HaloTag protein to quantify the fluorogenicity of the dye ligand. Normalized absorption (bold lines) and emission (dashed lines) of JF635i-HaloTag ligand in DMEM containing 10% FBS were measured in the presence or the absence of HaloTag protein. (C) JF635i-HaloTag ligand was synthesized in four steps from 6-tert-butoxycarbonylsilafluorescein ditriflate. The methyl ester-protected azetidines were introduced by palladium-catalyzed cross-coupling. Deprotection of the 6-tert-butyl ester S1 with TFA followed by amide coupling afforded intermediate S3, which was deprotected with NaOH to afford the JF635i-HaloTag ligand 4. (D) The pH titration assay showed that the fluorescence emission of JF635i-HaloTag ligand was stable above pH 3.8 (duplicate experiments; error bars represent standard deviation of the mean). (E) Representative images of U2OS cells expressing either H2B-HaloTag (left) or pDisplay-HaloTag (right) labeled with 200 nM JF635i-HaloTag ligand (JF635i-HTL) for 30 min and imaged directly without washing. (F) As positive control, we used U2OS cells expressing the same constructs as C, labeled with 200 nM cell-permeant JF635-HaloTag ligand (JF635-HTL). Images are displayed as a maximum intensity projection of a confocal image stack using Fiji. Scale bars: 50 µm.