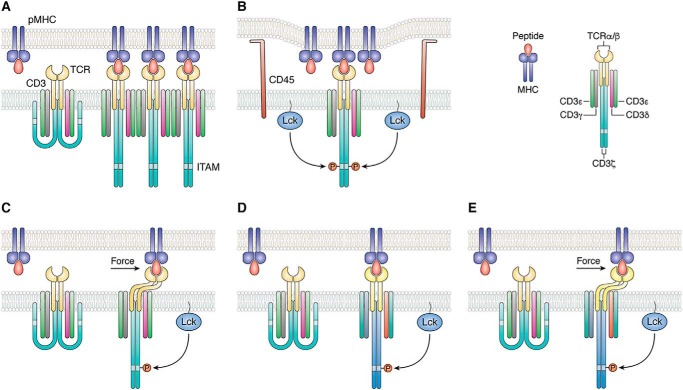
Figure 1.
Mechanisms of TCR activation. A, in the aggregation model, pMHC binding induces oligomerization of TCR–CD3 complexes. This clustering could increase the proximity of associated Lck molecules, resulting in activation of receptors in the aggregate by trans-autophosphorylation. B, segregation model proposes that TCR binding to pMHC induces zones of close contact at the T-cell–APC interface from which molecules with large ectodomains, such as the inhibitory tyrosine phosphatase CD45, are excluded. Segregation of CD45 favors phosphorylation of CD3 ITAMs by Lck. C, in the mechanosensing model, sliding of the T cell and APC membranes over each other during immune surveillance generates a mechanical force tangential to the T-cell surface that leads to dissociation of CD3 ITAMs from the T-cell membrane, thereby exposing them to phosphorylation by Lck. D, allosteric model postulates that pMHC binding to TCR induces long-range changes in TCR dynamics and/or conformation (represented by color changes in the TCR–CD3 complex) that are transmitted to the cytoplasmic tails of CD3 to expose ITAMs for phosphorylation. This transmission is mediated by allosteric sites in the TCR Cα and Cβ domains. E, in a unified model of TCR activation that combines mechanosensing (C) and allostery (D), mechanical force induces allosteric changes in TCR dynamics and/or conformation that propagate to CD3. Force amplifies allosteric communication between TCR and CD3 following pMHC ligation.