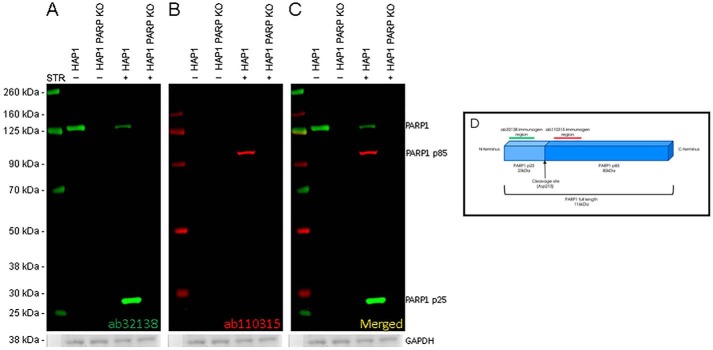
Figure 7.
Analysis of PARP1 cleavage by Western blotting. HAP1 WT and HAP1 PARP1 KO cells with (+) and without (−) staurosporine (STR) treatment were lysed, and 20 μg of total protein was loaded into a 4–12% Bis-Tris gel and run under the MOPS buffer system. The membrane was blocked for an hour using Odyssey blocking buffer (TBS) before incubation with rabbit anti-PARP1 antibody (ab32138) and mouse anti-cleaved PARP1 ab110315 at a 1:1000 dilution (9.7 μg/ml) and 1 μg/ml concentration, respectively. Antibody binding was detected using goat anti-rabbit IgG H&L (IRDye® 800CW) preadsorbed and goat anti-mouse IgG H&L (IRDye® 680RD) preadsorbed secondary antibodies at 1:20,000 dilution. A, full-length PARP1 was identified at 130 kDa by ab32138 (green) in HAP1 WT untreated lysates. Following treatment with staurosporine and cleavage of PARP1 in HAP1 WT cells, ab32138 detects a significantly weaker full-length PARP1 signal at 130 kDa alongside a new, stronger band at 28 kDa that represents the N-terminal cleavage product. No banding at either molecular weight is seen in treated or control HAP1 PARP1 knockout lysates. B, following treatment with staurosporine and cleavage of PARP1 in HAP1 WT cells, ab110315 (red) identifies the C-terminal cleavage product of PARP1 at 100 kDa. No banding is seen in the untreated WT control or in HAP1 PARP1 knockout lysates. C, overlay of both 800 and 70 nm displays clear identification of full-length PARP1 and cleavage products in staurosporine-treated HAP1 cells. Membranes were visualized using the Odyssey CLx imager with auto-intensity and 84-μm resolution. The membrane was then probed with an anti-GAPDH rabbit antibody conjugated to HRP (ab9385). Staining was developed for 20 min using GBOX XT-16 chemiluminescent imager. D, illustration of full-length PARP1 protein and associated cleavage products. Immunogen domains of ab32138 (green) and ab110315 (red) are displayed with the corresponding imaging channel color.