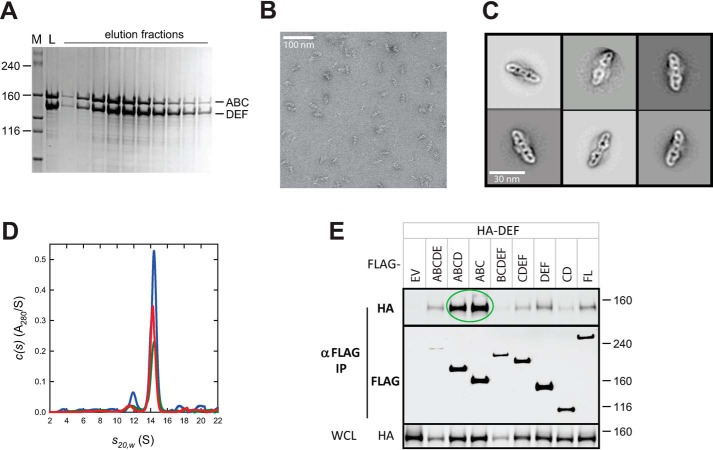
Figure 6.
Reconstitution of full-length neurofibromin dimers from ABC and DEF proteins. A, SDS-PAGE analysis of SEC of a equimolar mixture of ABC and DEF proteins. Size of molecular mass markers in lane M are noted in kilodaltons. Lane L represents the loaded material, and additional lanes are elution fractions across the column. B, transmission electron micrograph of reconstituted neurofibromin dimers from the ABC/DEF mixture. Scale bar (100 nm) is noted. C, representative 2D class averages from particles selected from multiple transmission electron microscopic micrographs. Scale bar (30 nm) is noted. D, sedimentation velocity absorbance c(s) profiles for equimolar mixtures of the N-terminal ABC and C-terminal DEF fragments of NF1 at 1.0 μm (green), 2.0 μm (red), and 4.5 μm (blue) based on data collected at 280 nm. Data at the highest concentration were collected using a 3-mm pathlength cell; standard 12 mm cells were used for the lower concentrations. E, Western blotting of co-immunoprecipitation of differentially epitope-tagged NF1 proteins in HEK293 cells. In this figure, HA-DEF protein was co-IPed with FLAG-tagged domains as noted. The top two gel sections are lysates purified with anti-FLAG antibodies and probed with antibodies to the HA or FLAG epitopes. The bottom section contains WCL probed with anti-HA antibodies. Molecular mass of standards are indicated on the right in kilodaltons. Circled regions are discussed in more detail in the text. EV, empty vector control; FL, full-length NF1.