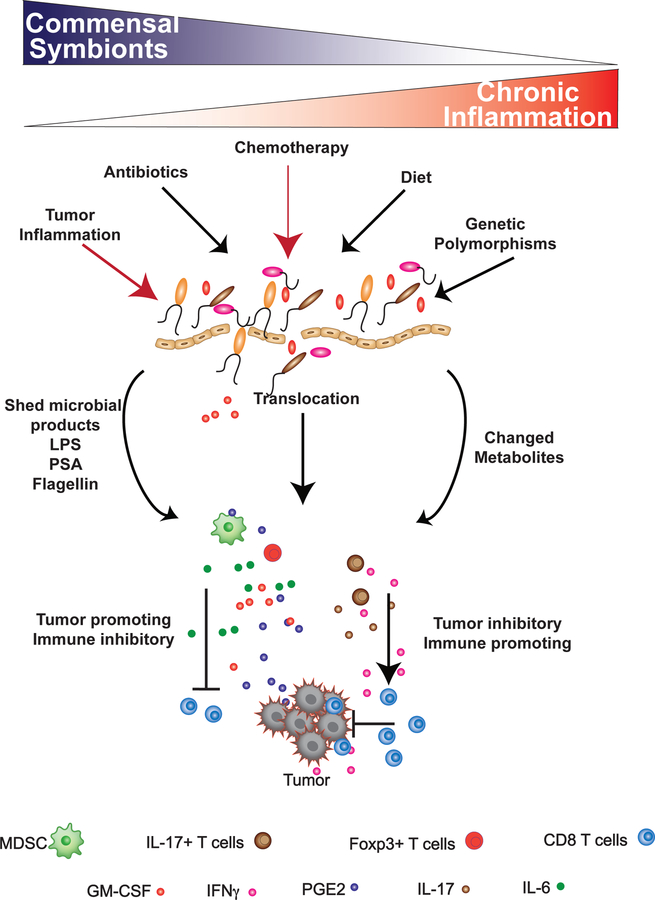
Figure 1: Loss of commensal equilibrium results in systemic inflammation and context-dependent modulation of extramucosal tumor progression.
Factors such as antibiotic usage, diet, or genetics (black arrows) can effectively decrease the biodiversity of the commensal microflora, leading to a higher incidence of chronic inflammation. In tumor-bearing individuals, cancer therapy and tumor-derived inflammation (red arrows) can also modulate the composition of the commensal ecosystem. A reduction in commensal biodiversity (dysbiosis), antibiotics, and chemotherapy can directly damage the integrity of the mucosal epithelium, resulting in bacterial translocation, commensal shedding of inflammatory products, such as LPS, PSA, and flagellin, and a change in metabolic output from the commensal ecosystem. This can lead to an enhancement in tumor-promoting inflammation due to the induction of IL-6, GM-CSF, and TGFβ: factors which enhance the recruitment of immune suppressive myeloid-derived suppressor cells and regulatory T cells into the tumor microenvironment. In the presence of these cells, the ability of T cells to effectively eliminate the tumor are inhibited. Translocation of commensals from mucosal surfaces can also induce the activation of IFNγ-producing Th-17 cells, which in certain contexts, can facilitate T cell-mediated killing of tumors.