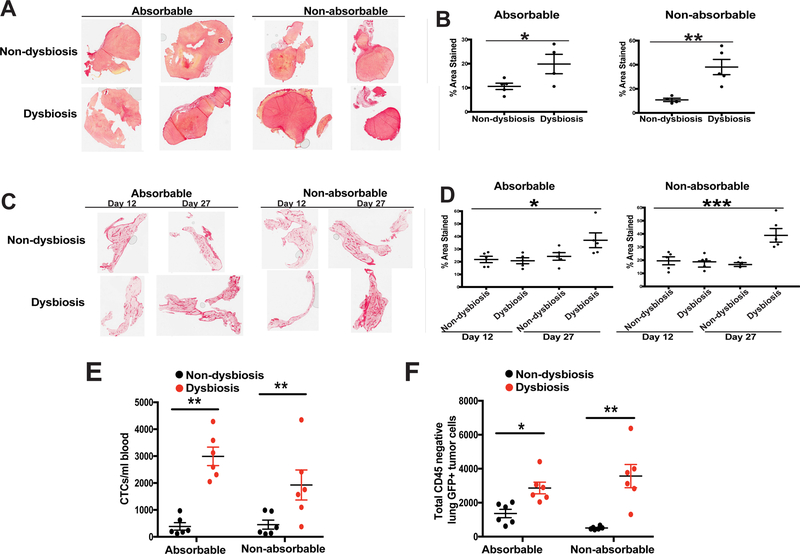
Figure 6. Direct targeting of gut commensals using non-absorbable antibiotics results in enhanced fibrosis and tumor cell dissemination in advanced tumor-bearing dysbiotic mice.
C57BL/6 mice were treated as described in Figure 2A. Half of the antibiotic-treated animals were gavaged with the previously-described antibiotic cocktail while the other half were gavaged with a cocktail of non-absorbable antibiotics that have minimal absorption from the gut. Non-dysbiotic animals from each group were gavaged with similar volumes of water per the administered antibiotic cocktail. A. Advanced BRPKp110 mammary tumors and C. normal tumor-adjacent mammary glands at early (day 12) and advanced (day 27) timepoints after tumor initiation were harvested from tumor-bearing mice, with or without established dysbiosis. Tissues were formalin-fixed and paraffin-embedded, and sections were stained with PicroSirius Red. Quantification of staining intensity was calculated using Image J software for both tumors (B) and mammary glands (D). E-F. GFP+ tumor cell dissemination was quantified in peripheral blood (E) and lung tissue (F) by flow cytometry. Data is represented as absolute number of GFP+CD45− cells of live, singlet cells. The anti-GFP gate was chosen based upon FMOs and a stained lung sample spiked with GFP+ BRPKp110 tumor cells. Representative of two independent experiments with 5 mice/group