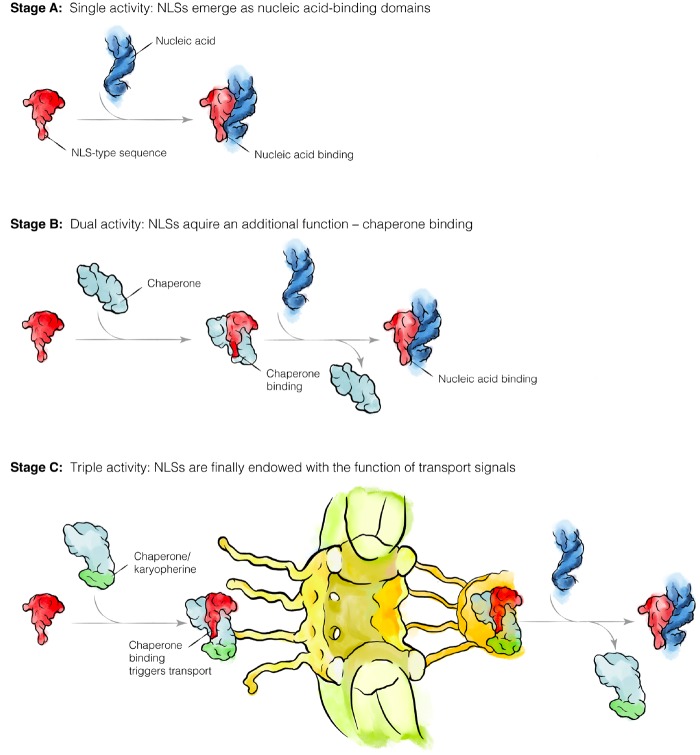
Fig. 5.
A hypothetical order of NLS evolution in eukaryotic proteins. In the modern eukaryotic cell, NLSs fulfil three biological activities: 1) they serve as signal peptides to direct protein transport into the nucleus; 2) during this protein transport, they recruit trafficking factors (karyopherins) that shield high positive charge of nucleic acid-binding domains (which prevents nonspecific interactions of NLS-containing proteins with other molecules in a cell); and 3) typically, NLSs reside within DNA- or RNA-binding domains of proteins and, therefore, NLSs mediate specific recognition of nucleic acids. Our model suggests that NLSs may have originally emerged as nucleic acid-binding domains in a cell lacking the nuclear–cytoplasmic separation (Stage A). Later, a chaperone emerged that shielded the highly positively charged nucleic acid-binding domains (Stage B). Finally, when cells got separated into the nucleus and the cytoplasm, this chaperone turned into a karyopherin when it acquired the additional capacity to mediate the long-distance trafficking of proteins across nuclear pores (Stage C). Thus, NLSs could initially emerge to help cellular proteins to recognize nucleic acids, and only later NLSs were endowed with an additional function of trafficking signals.