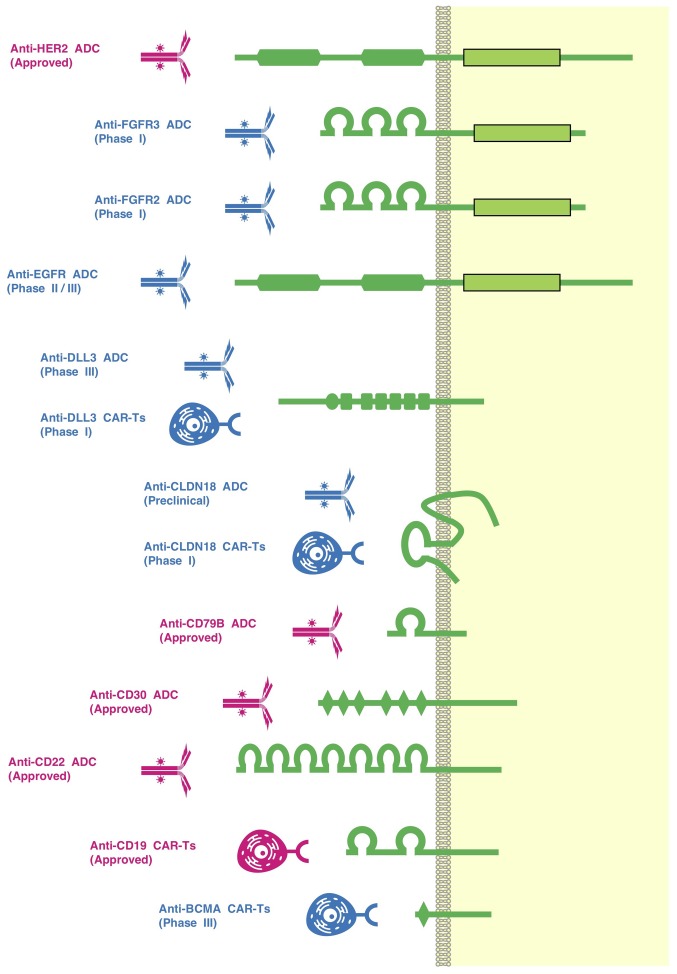
Figure 4.
ADCs and CAR-Ts. ADCs or CAR-Ts targeting BCMA, CD19, CD22, CD30, CD79B, CLDN18, DLL3, EGFR, FGFR2, FGFR3, HER2 and other transmembrane or GPI-anchored proteins have been developed as investigational drugs. Anti-CD19 CAR-Ts (axicabtagene ciloleucel and tisagenlecleucel), an anti-CD22 ADC (inotuzumab ozogamicin), an anti-CD30 ADC (brentuximab vedotin), an anti-CD79B ADC (polatuzumab vedotin) and an anti-HER2 ADC (trastuzumab emtansine) have been approved by the US Food and Drug Administration for the treatment of patients with cancer. A DLL3-targeting ADC, rovalpituzumab tesirine (Rova-T), is in phase III clinical trials for the treatment of patients with small-cell lung cancer (registration nos. NCT03033511 and NCT03061812). CLDN18, Claudin 18.2; ADC, antibody-drug conjugate; CAR-Ts, chimeric antigen receptor-modified T cells; BCMA, tumor necrosis factor receptor superfamily member 17; DLL3, delta-like canonical Notch ligand 3; EGFR, epidermal growth factor receptor; FGFR, fibroblast growth factor receptor.