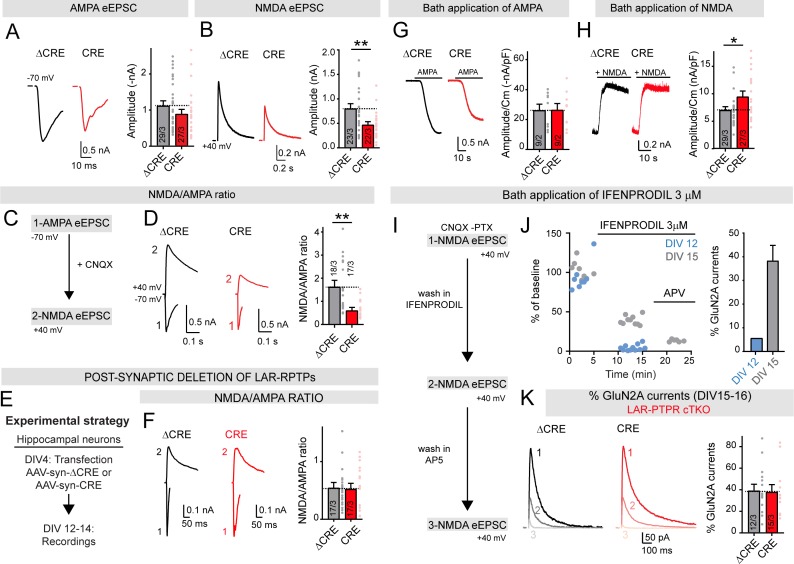
Figure 4. Deletion of LAR-RPTPs suppresses synaptic NMDAR- but not AMPAR-mediated responses in cultured hippocampal neurons.
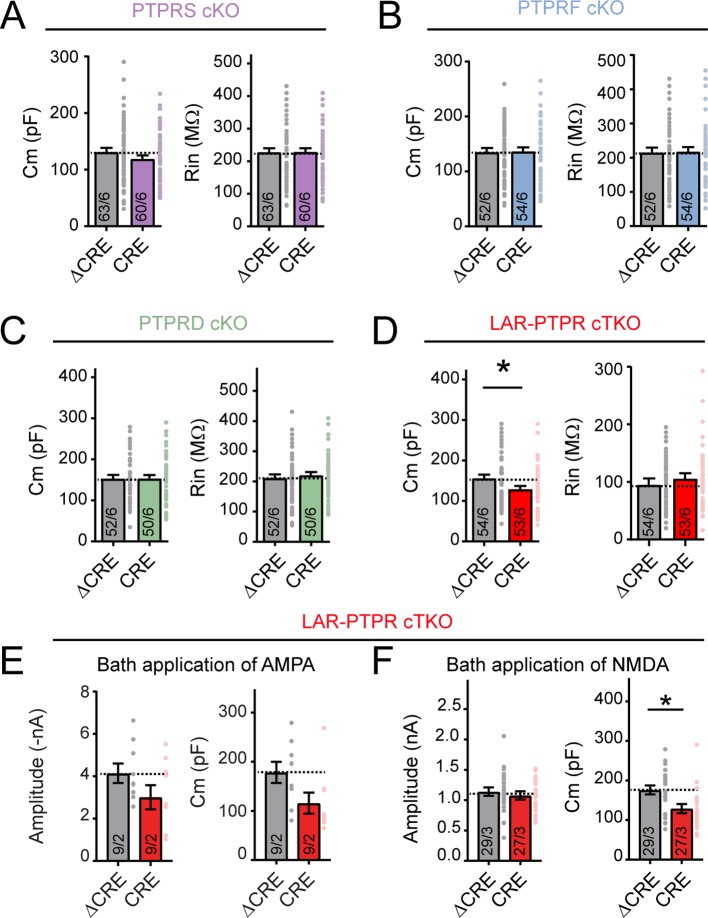
All experiments were performed with hippocampal neurons cultured from triple LAR-RPTP cKO mice. Neurons were infected with lentiviruses expressing Cre recombinase (CRE) or a non-functional mutant version of Cre recombinase (ΔCRE, used as control). (A) AMPAR-EPSC amplitudes recorded at −70 mV in presence of PTX are not significantly altered by deletion of all three LAR-RPTPs (left, representative traces; right, summary graphs). (B) NMDAR-EPSC amplitudes recorded at +40 mV in presence of PTX and CNQX are decreased by deletion of all three LAR-RPTPs (left, representative traces; right, summary graphs). (C) Experimental strategy for recording the NMDA/AMPA ratio in hippocampal cultures from triple LAR-RPTP cKO mice infected with ΔCRE (WT) or CRE (KO). (D) NMDAR/AMPAR-EPSC ratios are suppressed by deletion of LAR-RPTPs. AMPAR-EPSCs were recorded at −70 mV in the presence of PTX, and NMDAR-EPSCs were then recorded at +40 mV in the presence of CNQX (left, representative traces; right, summary graphs). (E) Experimental strategy for post-synaptic deletion of LAR-RPTPs in hippocampal cultures. Cultures were sparsely transfected with AAV-synapsin-ΔCRE-EGFP or AAV-synapsin-CRE plasmids at DIV4. Transfected cells expressing EGFP were patched at DIV12-14. (F) NMDA/AMPA ratio in EGFP positive neurons, expressing Cre or ΔCre recombinase, showing that post-synaptic deletion of LAR-RPTPs does not impair AMPAR- or NMDAR-mediated EPSCs. (G) AMPAR-responses elicited by bath-applied AMPA (1 μM) are unchanged by deletion of LAR-RPTPs (left, representative traces; right, summary graphs of peak current densities). (H) NMDAR-responses elicited by bath-applied NMDA (10 μM) and glycine (10 μM) are increased by deletion of LAR-RPTPs (left, representative traces; right, summary graphs of peak current densities). (I–K) Effect of a bath application of 3 μM Ifenprodil, a specific GluN2B blocker, on control hippocampal cultures at different maturation stages (DIV12, blue or DIV15, grey). (I) Schematic of experimental procedures: whole-cell recordings of hippocampal neurons were performed in the presence of CNQX and PTX in the bath to isolate NMDAR-EPSCs that were recorded at +40 mV. Ifenprodil (3 μM) and AP5 were sequentially added to the bath and NMDAR-EPSCs were recorded. (J) Sample traces of NMDAR-EPSCs (% of baseline) as a function of time. Application of Ifenprodil completely blocked NMDAR currents at DIV12 (in blue), but only partially reduced NMDAR currents at DIV15 (in grey), confirming that with maturation neurons switched from GluN2B only containing NMDARs to GluN2A containing NMDARs. Subsequent application of AP5 completely inhibited NMDAR-EPSCs at DIV15; right summary graph depicts the percentage of GluN2A currents at DIV 12 and DIV15. (K) Reduction of NMDAR-EPSCs induced by Ifenprodil was unchanged upon deletion of LAR-RPTPs, suggesting that the NMDAR composition does not depend on LAR-RPTPs. All data are means ± SEMs. Data comparing two conditions were analyzed by two-tailed unpaired Student’s t-test (for A, ΔCRE n = 29/3, CRE n = 27/3, p=0.1993; for B, ΔCRE n = 23/3, CRE n = 22/3, **p=0.0057; for D, ΔCRE n = 18/3, CRE n = 17/3, **p=0.0010; for F, ΔCRE n = 17/3, CRE n = 17/3, p=0.8726; for G, ΔCRE n = 9/2, CRE n = 9/2, p=0.9682; for H, ΔCRE n = 25/3, CRE n = 20/3, *p=0.0212, for K, ΔCRE n = 12/3, CRE n = 15/3, p=0.8891).