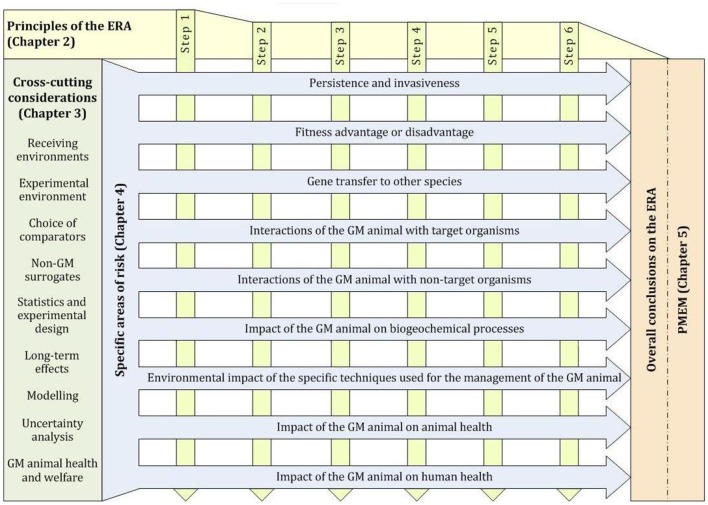
Figure 3.
Structure of the EFSA Guidance document on ERA of GM animals, which would apply to GD insects. In late 2018, EFSA received a new mandate from the EU Commission on GMOs engineered with gene drives (gene drive modified organisms) and their implications for RA methodologies. EFSA is requested to identify potential risks in terms of impact on human and animal health and the environment. EFSA is also asked to identify potential novel hazards and to determine whether the existing guidelines are adequate or whether there is a need for updated guidance. EFSA is not requested to develop guidelines for the RA of gene drive modified organisms. Thus, the current guidance on the ERA of GM plants and animals are still valid (EFSA Panel on Genetically Modified Organisms., 2010, 2013).