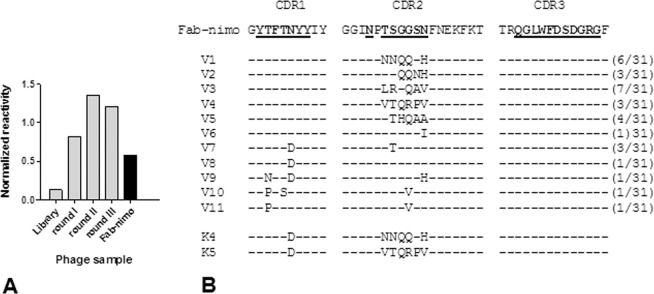
Figure 2.
Selection of mutated variants of nimotuzumab-derived Fab fragment by panning on EGF receptor extracellular region. CDR residues of the nimotuzumab heavy chain variable region were soft-randomized in a phage-displayed Fab library. Three rounds of phage selection were performed on the immobilized extracellular region of EGF receptor (erEGF-R) recombinant protein. The reactivity of rescued phage pools from the original library and from the different selection rounds was tested by ELISA on polyvinyl chloride microtiter plates coated with the human erEGF-R recombinant protein and with the anti-c-myc tag 9E10 mAb. The original phage-displayed Fab fragment derived from nimotuzumab (Fab-nimo) was included as a control. Bound phages were detected with an anti-M13 mAb conjugated to horseradish peroxidase. Normalized reactivities were estimated dividing the signal obtained with the human erEGF-R by the reference signal (measured with the anti-tag mAb) and allow the comparison of target recognition abilities of the different phage pools, despite variations in protein display levels. (A) Colony screening after the third selection round was used to choose 31 clones with the highest normalized reactivities. Their VH genes were sequenced (V1-V11), and the deduced protein CDR sequences were aligned. (B) The original CDR sequences of nimotuzumab are shown at the top, with those residues that were soft-randomized in the library highlighted in bold and underlined. Short lines represent conservation of the original residue at a given position. Numbers between parentheses indicate the frequencies of each mutated sequence among the set of 31 clones with the highest normalized reactivities. Two additional sequences (K4 and K5, bottom) were constructed by combining some of the selected mutations.