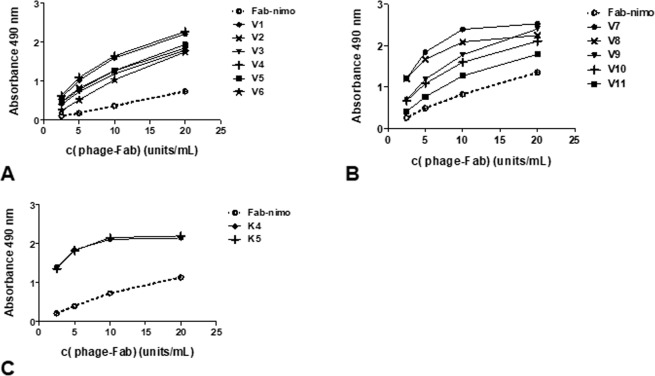
Figure 3.
Reactivity of phage-displayed mutated variants derived from nimotuzumab Fab. Purified phage particles were tested by ELISA on polyvinyl chloride microtiter plates coated with the recombinant protein comprising the extracellular region of human EGF receptor. Bound phages were detected with an anti-M13 mAb conjugated to horseradish peroxidase. Selected variants having mutations only in CDR2 (V1-V6) are represented in (A), while variants with replacements in both CDR1 and CDR2 (V7-V11) are shown in (B). Two new variants constructed by combining some of the selected mutations are represented in (C). The original nimotuzumab-derived phage-displayed fragment (Fab-nimo) was included as reference (dotted line). Dilutions of phage samples were calculated to reach equivalent concentrations of all the displayed proteins (display units/mL) according to a previous ELISA on polyvinyl chloride microtiter plates coated with the anti-tag antibody 9E10 to measure display levels.