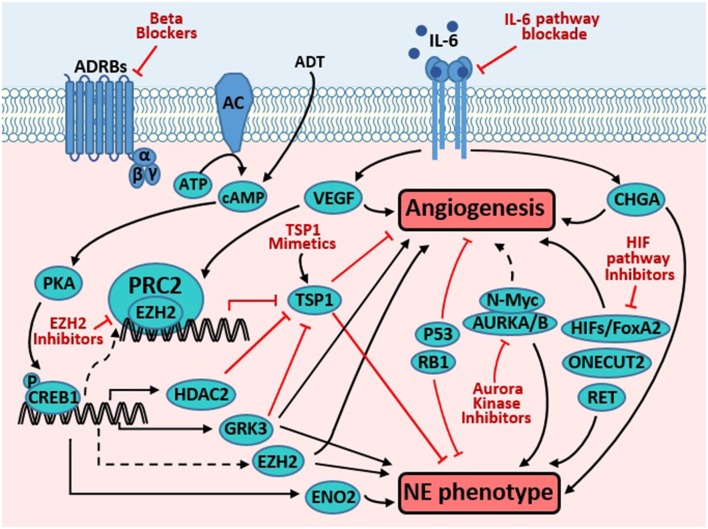
Figure 1.
Targeting molecules common to pathways promoting angiogenesis and neuroendocrine phenotype in prostate cancer. Androgen derivation therapy (ADT) elevates cAMP level, which activates PKA, resulting in phosphorylation and activation of CREB1. Activated CREB1 directly induces transcription of several genes involved in neuroendocrine differentiation (NED) and angiogenesis, such as VEGF, ENO2, GRK3, and HDAC2. VEGF is a potent pro-angiogenic factor, while ENO2 is a neuroendocrine marker. GRK3 promotes angiogenesis, NE marker expression, and prostate cancer progression. HDAC2 is critical for prostate cancer progression that is induced by chronical bio-behavioral stress and signals from beta adrenergic receptors (ADRBs). GRK3 and HDAC2 promotes angiogenesis, at least in part through downregulating TSP1. TSP1 is well-established as an anti-angiogenesis factor. Through unclear mechanisms, CREB1 activation enhances the PRC2 function of EZH2, which is critical for NED and angiogenesis induced by ADT. In endothelial cells, VEGF induces EZH2 expression and activity, which contributes to VEGF's action in promoting angiogenesis. Loss of p53 and RB1, alone or in cooperation, promote angiogenesis and NE phenotype through multiple mechanisms (detailed in text). IL-6 pathway activation enhances angiogenesis (through inducing VEGF) and NE phenotype (through inducing CHGA). AURKA interacts with N-Myc and regulates the stability of the latter, which promotes NED. AURKA and AURKB regulate angiogenesis in endothelial and neuroblastoma cells. HIF1A promotes angiogenesis through inducing VEGF. Moreover, it also cooperates with FoxA2 to promote NED and tumorigenesis. ONECUT2 has recently emerged as a master regulator of NED. Recent studies have also implicated receptor tyrosine kinase RET in regulating NED and angiogenesis. Novel strategies targeting the proteins and pathways that regulate both prominent phenotypes may be effective to treat NEPC (detailed in text).