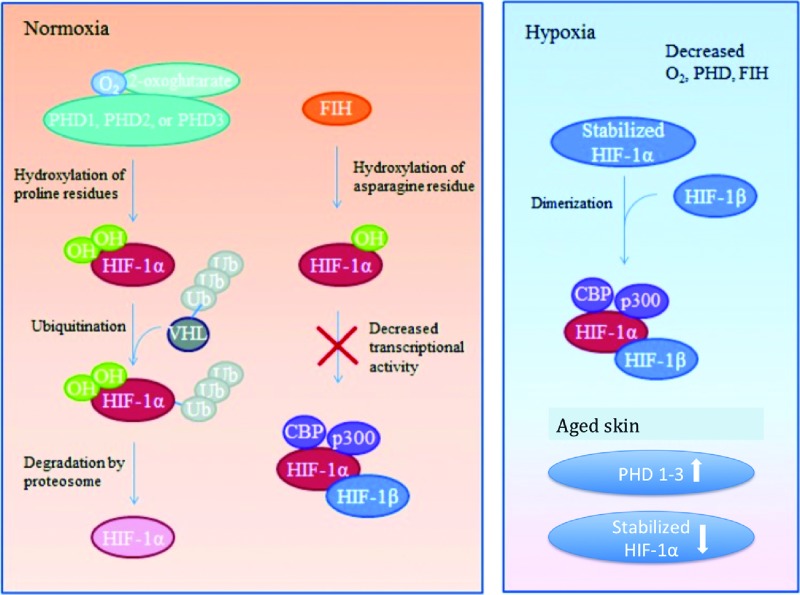
Figure 3.
HIF-1 regulation during normoxia, hypoxia. Under normoxic conditions HIF-1α is inhibited from binding with its coactivators by hydroxylation through PHD-1, -2, or -3 or FIH. PHD hydroxylates select proline residues on HIF-1α causing a conformational change that allows ubiquitination by a VHL ubiquitin–protein ligase complex followed by proteosomal degradation. FIH hydroxylates asparagine residues within HIF-1α, preventing interaction between HIF-1α and its coactivators. The hydroxylation described requires available oxygen. Under reduced oxygen conditions (hypoxia) HIF-1α is stabilized. With a dearth of available oxygen, hydroxylation of the HIF-1α subunit cannot occur and it readily binds with its coactivators. The stabilized HIF-1α subunit binds to HIF-1β subunit to form an active transcription factor. Active HIF-1 α/HIF-1β then bind to coactivators CBP and p300 to promote expression of downstream target genes. Aged skin PHD 1–3 levels are unregulated, leading to an increase in their prevalence and subsequent increase in HIF-1α degradation.59 FIH, factor inhibiting HIF; HIF-1α, hypoxia-inducible factor 1-alpha; PHDs, prolyl hydroxylase domain proteins; VHL, von Hippel–Lindau. Color images are available online.