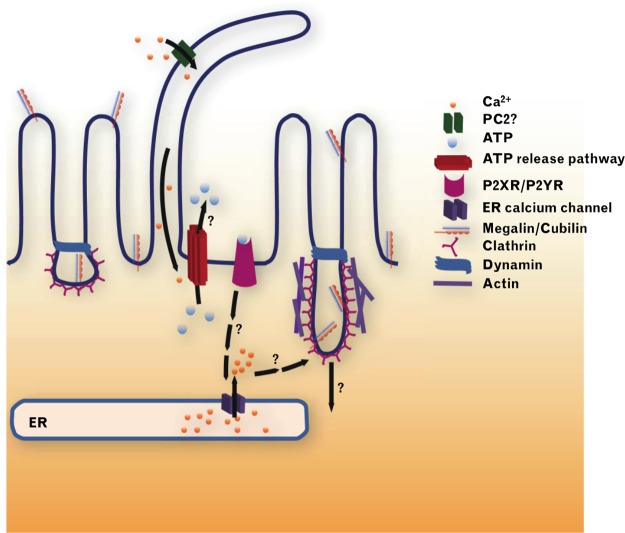
FIGURE 6.
Regulation of renal transport processes by flow-induced and cilium-mediated nucleotide release. Model of ciliary-activated release of ATP, which in turn activates P2X and P2Y receptors to increase intracellular calcium from endoplasmatic reticulum (ER) stores and stimulation of inositol trisphosphate (IP3) receptor-mediated enhancement of, in this example, apical endocytosis in the proximal tubule. 1) Exposure to flow-induced shear stress (FSS) bends the apical cilium, which causes entry of extracellular Ca2+ via a ciliary-localized cation channel [possibly polycystin-2 (PC2)] thereby increasing intracellular Ca2+ ([Ca2+]i). 2) Bending of the primary cilium causes release of ATP into the tubular lumen (via nucleotide transporters or other mechanisms) which in turn activates P2Y or P2X receptors in the apical membrane and increases [Ca2+]i. 3) The latter enhances the endocytic capacity at the base of microvilli, possibly by modulating actin dynamics to increase the size and volume of individual apical clathrin-coated pits. Addition of ATP bypasses the need for FSS in enhancing endocytic capacity. [Modified from Raghavan et al. (420) and Raghavan and Weisz (421), with permission from Wolters Kluwer Health, Inc.]